Sign In
to continue to Youth4work

information!
Study material
Mba-Master Of Business Administration

KSOU-Karnataka State Open University
MBA First Semester Managerial ...
This is sample paper of MBA 1st sem of Managerial Economics. The sections of the sample paper is as under:<br /> • Management Theory & Practice<br /> • Managerial Economics<br /> • Accounting for Managers<br /> • Organizational Behavior<br /> • Quantitative Techniques<br /> • Business Ethics & Values<br /> <br />

MCE-Mookambigai College of Engineering
Preceding Years Question Paper ...
This is the sample paper of Bharathiar University MBA I yr Quantitative Techniques Exam.In this paper ,it contains 8 questions and student have to complete the paper in 3 hrs.Student have to choose 5 questions from 8 qusetions which is of 20 marks each.Total question paper is of 100 marks.
MU-Madras University
industrial and labour law
YHREEEEEEEEEEEEEEE

AMSSOI-Andhra Mahila Sabha School of Informatics
organisational diagonistic model
An organizational model is a representation of an organization that helps us to understand more clearly and quickly what we are observing in organizations. Burke explains the many ways in which organizational models are useful (in Howard and Associates, 1994):<br /> <ul> <li> Models help to enhance our understanding of organizational behavior.</li> <li> Models help to categorize data about an organization.</li> <li> Models help to interpret data about an organization.</li> </ul> <br /> The models are presented in the chronological order in which they first appeared in the literature. The models reviewed in this section include:<br /> <ol> <li> Force Field Analysis (1951)</li> <li> Leavitt’s Model (1965)</li> <li> Likert System Analysis (1967)</li> <li> Open Systems Theory (1966)</li> <li> Weisbord’s Six-Box Model (1976)</li> <li> Congruence Model for Organization Analysis (1977)</li> <li> McKinsey 7S Framework (1981-82)</li> <li> Tichy’s Technical Political Cultural (TPC) Framework (1983)</li> <li> High-Performance Programming (1984)</li> <li> Diagnosing Individual and Group Behavior (1987)</li> <li> Burke-Litwin Model of Organizational Performance & Change (1992)</li> <li> Falletta’s Organizational Intelligence Model (2008)</li> </ol>
RIT-Roorkee Institute of Technology
consumer decision making
It gives you an idea how conumers decision can be effected.

BNC-Bihar National College
Lectures Notes & Study Materials Title
Lectures Notes & Study Materials Description
JBS-Jaypee Business School
Fiscalpolicyofindia
In economics and political science, fiscal policy is the use of government revenue collection (taxation) and expenditure (spending) to influence the economy.[1] The two main instruments of fiscal policy are government taxation and expenditure. Changes in the level and composition of taxation and government spending can impact the following variables in the economy:<br /> Aggregate demand and the level of economic activity;<br /> The pattern of resource allocation;<br /> The distribution of income.
MU-Magadh University
ANS COLLEGE
HEALTH
SGIR-Sunshine Group of Institutions Rajkot
BE & CG
MBA

AMSSOI-Andhra Mahila Sabha School of Informatics
IT Database concepts notes of Osmania University
<strong>Database systems concepts:</strong><br /> <br /> A database is a collection of related data. By data, we mean known facts that can be recorded and that have implicit meaning. For example, consider the names, telephone numbers and addresses of the people we know.<br /> <br /> <strong>A Database Management System (DBMS)</strong> is a collection of inter-related data and a set of programs to access those data. The primary goal of a DBMS is to provide an environment that is both convenient and efficient to use in retrieving and storing database information. DBMS is a general purpose software system that facilitates the processes of defining, constructing, manipulating and sharing databases among various users and applications. Defining a database involves specifying the data types, structures and constraints to the data to be stored in the database. The database definition or descriptive information is also stored in the database in the form of a database catalog or dictionary; it is called metadata. Constructing a database is the process of storing the data on some storage medium that is controlled by the DBMS. Manipulating the database includes functions such as querying the database to retrieve specific data, updating the database to reflect the real world changes and generating reports from the data. Sharing a database allows multiple users and programs to access the database simultaneously. Other important functions provided by the DBMS include protecting the database and maintaining it over a long period of time. Protection includes system protection against hardware or software malfunctions and security protection against unauthorized or malicious access. Maintenance includes allowing the system to evolve as requirements changes over time.<br /> <br /> <strong>Purpose of Database Systems</strong><br /> <br /> The typical file processing system is supported by a conventional operating system. Permanent records are stored in various files and different application programs are written to extract records from, and add records to, the appropriate files. Keeping organizational information in a file processing system has a number of disadvantages. <ul> <li> <strong>Data redundancy and inconsistency:</strong> Since different programmers create the files and application programs over a long period, the various files are likely to have different formats and the programs may be written in several programming languages. Moreover, the same information may be duplicated in several places (files). For example, the address and telephone number of a particular customer may appear in a file that consists of savings-account records and in a file that consists of checking-account records. This redundancy leads to higher storage and access cost. In addition, it may lead to data inconsistency; that is, the various copies of the same data may no longer agree. For example, a changed customer address may be reflected in savings-account records but not elsewhere in the system.</li> </ul> <br /> <br /> <ul> <li> <strong>Difficulty in accessing data</strong>: Suppose that one of the bank officers needs to find out the names of all customers who live within a particular postal-code area. The officer asks the data-processing department to generate such a list. Because the designers of the original system did not anticipate this request, there is no application program on hand to meet it. There is, however, an application program to generate the list of all customers. The bank officer has now two choices: either obtain the list of all customers and extract the needed information manually or ask a system programmer to write the necessary application program. Both alternatives are obviously unsatisfactory. Suppose that such a program is written, and that, several days later, the same officer needs to trim that list to include only those customers who have an account balance of $10,000 or more. As expected, a program to generate such a list does not exist. Again, the officer has the preceding two options, neither of which is satisfactory.The point here is that conventional file-processing environments do not allow needed data to be retrieved in a convenient and efficient manner. More responsive data-retrieval systems are required for general use.</li> </ul> <br /> <br /> <ul> <li> <strong>Data isolation:</strong> Because data are scattered in various files, and files may be in different formats, writing new application programs to retrieve the appropriate data is difficult.</li> </ul> <br /> <br /> <ul> <li> <strong>Integrity problems:</strong> The data values stored in the database must satisfy certain types of consistency constraints. For example, the balance of a bank account may never fall below a prescribed amount (say, $25). Developers enforce these constraints in the system by adding appropriate code in the various application programs. However, when new constraints are added, it is difficult to change the programs to enforce them. The problem is compounded when constraints involve several data items from different files.</li> </ul> <br /> <br /> <ul> <li> <strong>Atomicity problems</strong>. A computer system, like any other mechanical or electrical device, is subject to failure. In many applications, it is crucial that, if a failure occurs, the data be restored to the consistent state that existed prior to the failure. Consider a program to transfer $50 from account A to account B. If a system failure occurs during the execution of the program, it is possible that the $50 was removed from account A, but was not credited to account B, resulting in an inconsistent database state. Clearly, it is essential to database consistency that either both the credit and debit occur, or that neither occur. That is, the funds transfer must be atomic—it must happen in its entirety or not at all. It is difficult to ensure atomicity in a conventional file-processing system.</li> </ul> <br /> <br /> <ul> <li> <strong>Concurrent-access anomalies:</strong> For the sake of overall performance of the system and faster response, many systems allow multiple users to update the data simultaneously. In such an environment, interaction of concurrent updates may result in inconsistent data. Consider bank account A, containing $500. If two customers withdraw funds (say $50 and $100 respectively) from account A at about the same time, the result of the concurrent executions may leave the account in an incorrect (or inconsistent) state. Suppose that the programs executing on behalf of each withdrawal read the old balance, reduce that value by the amount being withdrawn, and write the result back. If the two programs run concurrently, they may both read the value $500, and write back $450 and $400, respectively. Depending on which one writes the value last, the account may contain either $450 or $400, rather than the correct value of $350. To guard against this possibility, the system must maintain some form of supervision. But supervision is difficult to provide because data may be accessed by many different application programs that have not been coordinated previously.</li> </ul> <br /> <br /> <ul> <li> <strong>Security problems:</strong> Not every user of the database system should be able to access all the data. For example, in a banking system, payroll personnel need to see only that part of the database that has information about the various bank employees. They do not need access to information about customer accounts. But, since application programs are added to the system in an ad hoc manner, enforcing such security constraints is difficult.</li> </ul>

AMSSOI-Andhra Mahila Sabha School of Informatics
computer security threats note ...
<strong>computer security</strong><br /> Security of any kind of information like personal information or even the security of a computer is very important in the present world. With computers being most used and most trusted the security of a computer is one of the major concerns of its users.<br /> <br /> Computer Security is the branch of technology known as information security. It is mainly applied to computers and networks. As the name says it is about the protection of information, property, corruption etc while keeping it accessible for its intended users.<br /> <br /> <br /> <br /> A hardware device allows the user to login and logout and set access privileges. The hardware protects the operating system image and file access privileges from being tampered. Illegal access can be detected by a mal program or user can be detected by the current state of the user by the harddisk or DVD controllers.<br /> <br /> <br /> <br /> In computer systems Access control lists (ACL’s) and capabilities are the two fundamental ways of enforcing privilege separation. The problems of insecure semantics and one at a time access to an object of ACL can be solved by using capabilities. While capabilities are being used by research operating systems, ACL’s are being used by commercial operating systems.<br /> <br /> Applications:-<br /> <br /> Computer Security is widely being used and is very important for aviation. Aviation industry is especially important because it usually involves the lives of human beings, cost included for expensive equipment,infrastructure,cargo,shipment etc.Sabotage,espionage,terrorist threats, mechanical malfunctioning, human error are just few of the possibilities when there is no computer security. Power failure, blown fuses, lightning all cause the computer system to disable as it is dependent on electricity.<br /> <br /> <strong>Security Threats can occur at</strong><br /> Network level<br /> Data level<br />
GIMR-Godavari Institute of Management and Research
html and website management
all chapter notes

AAG-Academy of Animation and Gaming
Managerial Economics Exam sam ...
This is the sample paper of Managerial Economics Exam sample paper.In this paper students should complete this paper in 3 hours.This university is fastly growing distance education university.This paper is of 75 marks.This paper contains 3 sections.first section is of 45 marks,second section is of 20 marks and third section is of 10 marks.

AAG-Academy of Animation and Gaming
Accounting for Managers exam s ...
This is the sample paper of Accounting for Managers exam sample paper.In this paper students should complete this paper in 3 hours.This university is fastly growing distance education university.This paper contains 3 sections.first section is of 45 marks,second section is of 20 marks and third section is of 10 marks.

AAG-Academy of Animation and Gaming
Business Ethics and Values Exa ...
This is the sample paper of Business Ethics and Values Exam sample paper.In this paper students should complete this paper in 3 hours.In this subject explains that what is the values and ethics of bussiness.This university shortly called KSOU.and this plays an important role to develop higher education.
MH-Miranda House
plant ecology general
Plant ecology is a subdiscipline of ecology. There are three main parts: (1) the distribution and abundance of plants, the effects of environmental factors upon the abundance of plants, and (3) the interactions among and between members of plant species in particular and other organisms in general.[1] An example of the first might be the study of the distribution of temperate deciduous forests in North America. An example of the second might be the effects of drought or flooding upon plant survivial. Examples of the third might included competition among desert plants for water, or effects of herds of grazing animals upon the composition of grasslands.<br /> <br /> <br /> Tropical plant community on Diego Garcia.<br /> A global overview of the Earth's major vegetation types is provided by O.W. Archibold.[2] He recognizes 11 major vegetation types: tropical forests, tropical savnnas, arid regions (deserts), Mediterranean ecosystems, temperate forest ecosystems, temperate grasslands, coniferous forests, tundra (both polar and high mountain), terrestrial wetlands, freshwater ecosystems and coastal/marine systems. This breadth of topics shows the complexity of plant ecology, since it includes plants from floating single-celled algae up to large canopy forming trees.<br /> One feature that defines plants is photosynthesis. One of the most important aspects of plant ecology is the role plants have played in creating the oxygenated atmosphere of earth, an event that occurred some 2 billion years ago. It can be dated by the deposition of banded iron formations, distinctive sedimentary rocks with large amounts of iron oxide. At the same time, plants began removing carbon dioxide from the atmosphere, thereby initiating the process of controlling Earth's climate. A long term trend of the Earth has been toward increasing oxygen and decreasing carbon dioxide, and many other events in the Earths history, like the first movement of life onto land, are likely tied to this sequence of events.[1]<br /> One of the early classic books on plant ecology was written by Weaver and Clements.[3] It talks broadly about plant communities, and particulalry the importance of forces like competition and processes like succession. Although some of the terminology is dated, this important book can still often be obtained in used book stores.<br /> Plant ecology can also be divided by levels of organization including plant ecophysiology, plant population ecology, community ecology, ecosystem ecology, landscape ecology and biosphere ecology.[1][4]<br /> The study of plants and vegetation is complicated by their form. First, most plants are rooted in the soil, which makes it difficult to observe and measure nutrient uptake and species interactions. Second, plants often reproduce vegetatively, that is asexually, in a way that makes it difficult to distinguish individual plants. Indeed, the very concept of an individual is doubtful, since even a tree may be regared as a large collection of linked meristems.[5] Hence, plant ecology and animal ecology have different styles of approach to problems that involve processes like reproduction, dispersal and mutualism. Some plant ecologists have placed considerable emphasis upon trying to treat plant populations as if they were animal populations, focusing on population ecology.[6] Many other ecologists believe that while it is useful to draw upon population ecology to solve certain scientific problems, plants demand that ecologists work with multiple perspectives, appropriate to the problem, the scale and the situation.[1]<br /> Contents [hide]<br /> 1 Distribution<br /> 2 Biological interactions<br /> 2.1 Competition<br /> 2.2 Mutualism<br /> 2.3 Herbivory<br /> 3 Other topics<br /> 3.1 Abundance<br /> 3.2 Colonisation and local extinction<br /> 3.3 Life forms<br /> 3.4 Strategies<br /> 3.5 Reproduction<br /> 4 See also<br /> 5 References<br /> 5.1 Further reading<br /> [edit]Distribution<br /> <br /> Main articles: Phytogeography and Species distribution<br /> <br /> <br /> World biomes are based upon the type of dominant plant.<br /> Plant communities are broadly distributed into biomes based on the form of the dominant plant species. For example, grasslands are dominated by grasses, while forests are dominated by trees. Biomes are determined by regional climates, mostly temperature and precipitation, and follow general latitudinal trends. Within biomes, there may be many ecological communities, which are impacted not only by climate and a variety of smaller-scale features, including soils, hydrology, and disturbance regime. Biomes also change with elevation, high elevations often resembling those found at higher latitudes.<br /> [edit]Biological interactions<br /> <br /> Main article: Biological interaction<br /> [edit]Competition<br /> Main article: Competition (biology)<br /> Plants, like most life forms, require relatively few basic elements: Carbon, hydrogen, oxygen, nitrogen, phosphorus and sulphur; hence they are known as CHNOPS life forms. There are also lesser elements needed as well, frequently termed micronutrients, such as magnesium and sodium. When plants grow in close proximity, the may deplete supplies of these elements and have a negative impact upon neighbours. In many cases (perhaps most) the negative effects upon neighbours arise from competition for light, with larger plants shading smaller plants. In other cases, there may be competition below ground for water, nitrogen, or phosphorus. To detect and measure competition, experiments are necessary; these experiments require removing neighbours, and measuring responses in the remaining plants.[7] Many such studies are required before useful generalizations can be drawn.<br /> Overall, it appears that light is the most important resource for which plants complete, and the increase in plant height over evolutionary time likely reflects selection for taller plants to better intercept light. Many plant communities are therefore organized into hierarchies based upon the relative competitive abilties for light.[7] In some systems, particuarly infertile or arid systems, below ground competition may be more significant.[8] Along natural gradients of soil fertility, it is likely that the ratio of above ground to below ground competition changes, with higher above ground competition in the more fertile soils[9][10]. Plants that are relatvely weak competitors may escape in time (by surviving as buried seeds) or in space (by dispsersing to a new location away from strong competitors.)<br /> In principle, it is possible to examine competition at the level of the limiting resources if a detailed knowledge of the physiological processes of the competing plants is available. However, in most terrestrial ecological studies, there is only little information on the uptake and dynamics of the resources that limit the growth of different plant species, and, instead, competition is inferred from observed negative effects of neighbouring plants without knowing precisely which resources the plants were competing for. In certain situations, plants may compete for a single growth-limiting resource, perhaps for light in agricultural systems with sufficient water and nutrients, or in dense stands of marsh vegetation, but in many natural ecosystems plants may be colimited by several resources, e.g. light, phosphorus and nitrogen at the same time.[11]<br /> There are therefore many details that remain to be uncovered, particularly the kinds of competition that arise in natural plant communities, the specific resource(s), the relative importance of different resources, and the role of other factors like stress or disturbance in regulating the importance of competition.[12][1]<br /> [edit]Mutualism<br /> Main article: Mutualism<br /> Mutualism is defined as an interaction "between two species or individuals that is beneficial to both". Probably the most widespread example in plants is the mutual beneficial relationship between plants and fungi, known as mycorrhizae. The plant is assisted with nutrient uptake, while the the fungus receives carbohydrates. Some the the earliest known fossil plants even have fossil mycorrhizae on their rhizomes.[1]<br /> The flowering plants are a group that have evolved by using two major mutualisms. First, flowers are pollinated by insects. This relationship seems to have its origins in beetles feeding on primitive flowers, eating pollen and also acting (unwittingly) as pollinators. Second, fruits are eaten by animals, and the animals then disperse the seeds. Thus, the flowering plants actually have three major types of mutualism, since most higher plants also have mycorrhizae.[1]<br /> Plants may also have beneficial effects upon one another, but this is less common. Examples might include "nurse plants" whose shade allows young cacti to establish. Most examples of mutualism, however, are largely beneficial to only one of the partners, and may not really be true mutualism. The term used for these more one-sided relationships, which are mostly beneficial to one participant, is facilitation. Facilitation among neighboring plants may act by reducing the negative impacts of a stressful environment.[13] In in general, facilitation is more likely to occur in physically stressful environments than in favorable environments, where competition may be the most important interaction among species.[14]<br /> Commensalism is similar to faciliation, in that one plant is mostly exploiting another. A familiar example is the ephiphytes which grow on branches of tropical trees, or even mosses which grow on trees in deciduous forests.<br /> It is important to keep track of the benfits received by each species to determine the appropriate term. Although people are often fascinated by unusual examples, it is important to remember that in plants, the main mutualisms are mycorrhizae, pollination, and seed dispersal. [1]<br /> [edit]Herbivory<br /> Main articles: Herbivory and Plant defense against herbivory<br /> An important ecological function of plants is that they produce organic compounds for herbivores[15] in the bottom of the food web. A large number of plant traits, from thorns to chemical defenses, can be related to the intensity of herbivory. Large herbivores can also have many effects on vegetation. These include removing selected species, creating gaps for regeneration of new individuals, recycling nutrients, and dispersing seeds. Certain ecosystem types, such as grasslands, may be dominated by the effects of large herbivores, although fire is also an equally important factor in this biome. In few cases, herbivores are capable of nearly removing all the vegetation at a site (for example, geese in the Hudson Bay Lowlands of Canada, and nutria in the marshes of Louisiana[16]) but normally heribovres have a more selective impact, particualry when large predators control the abundance of herbivores. The usual method of studying the effects of herbivores is to build exclosures, where they cannot feed, and compare the plant communites in the exclosures to those outisde over many years. Often such long term experiments show that herbivores have a significant effect upon the species that make up the plant community. [1]<br /> [edit]Other topics<br /> <br /> [edit]Abundance<br /> Main article: Abundance (ecology)<br /> The ecological success of a plant species in a specific environment may be quantified by its abundance, and depending on the life form of the plant different measures of abundance may be relevant, e.g. density, biomass, or plant cover.<br /> The change in the abundance of a plant species may be due to both abiotic factors, e.g. climate change, or biotic factors, e.g herbivory or interspecific competition.<br /> [edit]Colonisation and local extinction<br /> Main article: Biogeography<br /> See also: Colonisation (biology), Biological dispersal, Seed dispersal, Local extinction, and Soil seed bank<br /> Whether a plant species is present at a local area depends on the processes of colonisation and local extinction. The probaility of colonisation decreases with distance to neighboring habitats where the species is present and increases with plant abundance and fecundity in neighboring habitats and the dispersal distance of the species. The probability of local extinction decreases with abundance (both living plants and seeds in the soil seed bank).<br />
MH-Miranda House
plant ecology general
Plant ecology is a subdiscipline of ecology. There are three main parts: (1) the distribution and abundance of plants, the effects of environmental factors upon the abundance of plants, and (3) the interactions among and between members of plant species in particular and other organisms in general.[1] An example of the first might be the study of the distribution of temperate deciduous forests in North America. An example of the second might be the effects of drought or flooding upon plant survivial. Examples of the third might included competition among desert plants for water, or effects of herds of grazing animals upon the composition of grasslands.<br /> <br /> <br /> Tropical plant community on Diego Garcia.<br /> A global overview of the Earth's major vegetation types is provided by O.W. Archibold.[2] He recognizes 11 major vegetation types: tropical forests, tropical savnnas, arid regions (deserts), Mediterranean ecosystems, temperate forest ecosystems, temperate grasslands, coniferous forests, tundra (both polar and high mountain), terrestrial wetlands, freshwater ecosystems and coastal/marine systems. This breadth of topics shows the complexity of plant ecology, since it includes plants from floating single-celled algae up to large canopy forming trees.<br /> One feature that defines plants is photosynthesis. One of the most important aspects of plant ecology is the role plants have played in creating the oxygenated atmosphere of earth, an event that occurred some 2 billion years ago. It can be dated by the deposition of banded iron formations, distinctive sedimentary rocks with large amounts of iron oxide. At the same time, plants began removing carbon dioxide from the atmosphere, thereby initiating the process of controlling Earth's climate. A long term trend of the Earth has been toward increasing oxygen and decreasing carbon dioxide, and many other events in the Earths history, like the first movement of life onto land, are likely tied to this sequence of events.[1]<br /> One of the early classic books on plant ecology was written by Weaver and Clements.[3] It talks broadly about plant communities, and particulalry the importance of forces like competition and processes like succession. Although some of the terminology is dated, this important book can still often be obtained in used book stores.<br /> Plant ecology can also be divided by levels of organization including plant ecophysiology, plant population ecology, community ecology, ecosystem ecology, landscape ecology and biosphere ecology.[1][4]<br /> The study of plants and vegetation is complicated by their form. First, most plants are rooted in the soil, which makes it difficult to observe and measure nutrient uptake and species interactions. Second, plants often reproduce vegetatively, that is asexually, in a way that makes it difficult to distinguish individual plants. Indeed, the very concept of an individual is doubtful, since even a tree may be regared as a large collection of linked meristems.[5] Hence, plant ecology and animal ecology have different styles of approach to problems that involve processes like reproduction, dispersal and mutualism. Some plant ecologists have placed considerable emphasis upon trying to treat plant populations as if they were animal populations, focusing on population ecology.[6] Many other ecologists believe that while it is useful to draw upon population ecology to solve certain scientific problems, plants demand that ecologists work with multiple perspectives, appropriate to the problem, the scale and the situation.[1]<br /> Contents [hide]<br /> 1 Distribution<br /> 2 Biological interactions<br /> 2.1 Competition<br /> 2.2 Mutualism<br /> 2.3 Herbivory<br /> 3 Other topics<br /> 3.1 Abundance<br /> 3.2 Colonisation and local extinction<br /> 3.3 Life forms<br /> 3.4 Strategies<br /> 3.5 Reproduction<br /> 4 See also<br /> 5 References<br /> 5.1 Further reading<br /> [edit]Distribution<br /> <br /> Main articles: Phytogeography and Species distribution<br /> <br /> <br /> World biomes are based upon the type of dominant plant.<br /> Plant communities are broadly distributed into biomes based on the form of the dominant plant species. For example, grasslands are dominated by grasses, while forests are dominated by trees. Biomes are determined by regional climates, mostly temperature and precipitation, and follow general latitudinal trends. Within biomes, there may be many ecological communities, which are impacted not only by climate and a variety of smaller-scale features, including soils, hydrology, and disturbance regime. Biomes also change with elevation, high elevations often resembling those found at higher latitudes.<br /> [edit]Biological interactions<br /> <br /> Main article: Biological interaction<br /> [edit]Competition<br /> Main article: Competition (biology)<br /> Plants, like most life forms, require relatively few basic elements: Carbon, hydrogen, oxygen, nitrogen, phosphorus and sulphur; hence they are known as CHNOPS life forms. There are also lesser elements needed as well, frequently termed micronutrients, such as magnesium and sodium. When plants grow in close proximity, the may deplete supplies of these elements and have a negative impact upon neighbours. In many cases (perhaps most) the negative effects upon neighbours arise from competition for light, with larger plants shading smaller plants. In other cases, there may be competition below ground for water, nitrogen, or phosphorus. To detect and measure competition, experiments are necessary; these experiments require removing neighbours, and measuring responses in the remaining plants.[7] Many such studies are required before useful generalizations can be drawn.<br /> Overall, it appears that light is the most important resource for which plants complete, and the increase in plant height over evolutionary time likely reflects selection for taller plants to better intercept light. Many plant communities are therefore organized into hierarchies based upon the relative competitive abilties for light.[7] In some systems, particuarly infertile or arid systems, below ground competition may be more significant.[8] Along natural gradients of soil fertility, it is likely that the ratio of above ground to below ground competition changes, with higher above ground competition in the more fertile soils[9][10]. Plants that are relatvely weak competitors may escape in time (by surviving as buried seeds) or in space (by dispsersing to a new location away from strong competitors.)<br /> In principle, it is possible to examine competition at the level of the limiting resources if a detailed knowledge of the physiological processes of the competing plants is available. However, in most terrestrial ecological studies, there is only little information on the uptake and dynamics of the resources that limit the growth of different plant species, and, instead, competition is inferred from observed negative effects of neighbouring plants without knowing precisely which resources the plants were competing for. In certain situations, plants may compete for a single growth-limiting resource, perhaps for light in agricultural systems with sufficient water and nutrients, or in dense stands of marsh vegetation, but in many natural ecosystems plants may be colimited by several resources, e.g. light, phosphorus and nitrogen at the same time.[11]<br /> There are therefore many details that remain to be uncovered, particularly the kinds of competition that arise in natural plant communities, the specific resource(s), the relative importance of different resources, and the role of other factors like stress or disturbance in regulating the importance of competition.[12][1]<br /> [edit]Mutualism<br /> Main article: Mutualism<br /> Mutualism is defined as an interaction "between two species or individuals that is beneficial to both". Probably the most widespread example in plants is the mutual beneficial relationship between plants and fungi, known as mycorrhizae. The plant is assisted with nutrient uptake, while the the fungus receives carbohydrates. Some the the earliest known fossil plants even have fossil mycorrhizae on their rhizomes.[1]<br /> The flowering plants are a group that have evolved by using two major mutualisms. First, flowers are pollinated by insects. This relationship seems to have its origins in beetles feeding on primitive flowers, eating pollen and also acting (unwittingly) as pollinators. Second, fruits are eaten by animals, and the animals then disperse the seeds. Thus, the flowering plants actually have three major types of mutualism, since most higher plants also have mycorrhizae.[1]<br /> Plants may also have beneficial effects upon one another, but this is less common. Examples might include "nurse plants" whose shade allows young cacti to establish. Most examples of mutualism, however, are largely beneficial to only one of the partners, and may not really be true mutualism. The term used for these more one-sided relationships, which are mostly beneficial to one participant, is facilitation. Facilitation among neighboring plants may act by reducing the negative impacts of a stressful environment.[13] In in general, facilitation is more likely to occur in physically stressful environments than in favorable environments, where competition may be the most important interaction among species.[14]<br /> Commensalism is similar to faciliation, in that one plant is mostly exploiting another. A familiar example is the ephiphytes which grow on branches of tropical trees, or even mosses which grow on trees in deciduous forests.<br /> It is important to keep track of the benfits received by each species to determine the appropriate term. Although people are often fascinated by unusual examples, it is important to remember that in plants, the main mutualisms are mycorrhizae, pollination, and seed dispersal. [1]<br /> [edit]Herbivory<br /> Main articles: Herbivory and Plant defense against herbivory<br /> An important ecological function of plants is that they produce organic compounds for herbivores[15] in the bottom of the food web. A large number of plant traits, from thorns to chemical defenses, can be related to the intensity of herbivory. Large herbivores can also have many effects on vegetation. These include removing selected species, creating gaps for regeneration of new individuals, recycling nutrients, and dispersing seeds. Certain ecosystem types, such as grasslands, may be dominated by the effects of large herbivores, although fire is also an equally important factor in this biome. In few cases, herbivores are capable of nearly removing all the vegetation at a site (for example, geese in the Hudson Bay Lowlands of Canada, and nutria in the marshes of Louisiana[16]) but normally heribovres have a more selective impact, particualry when large predators control the abundance of herbivores. The usual method of studying the effects of herbivores is to build exclosures, where they cannot feed, and compare the plant communites in the exclosures to those outisde over many years. Often such long term experiments show that herbivores have a significant effect upon the species that make up the plant community. [1]<br /> [edit]Other topics<br /> <br /> [edit]Abundance<br /> Main article: Abundance (ecology)<br /> The ecological success of a plant species in a specific environment may be quantified by its abundance, and depending on the life form of the plant different measures of abundance may be relevant, e.g. density, biomass, or plant cover.<br /> The change in the abundance of a plant species may be due to both abiotic factors, e.g. climate change, or biotic factors, e.g herbivory or interspecific competition.<br /> [edit]Colonisation and local extinction<br /> Main article: Biogeography<br /> See also: Colonisation (biology), Biological dispersal, Seed dispersal, Local extinction, and Soil seed bank<br /> Whether a plant species is present at a local area depends on the processes of colonisation and local extinction. The probaility of colonisation decreases with distance to neighboring habitats where the species is present and increases with plant abundance and fecundity in neighboring habitats and the dispersal distance of the species. The probability of local extinction decreases with abundance (both living plants and seeds in the soil seed bank).<br />
MH-Miranda House
DNA replication general
DNA replication<br /> From Wikipedia, the free encyclopedia<br /> <br /> <br /> DNA replication. The double helix is unwound and each strand acts as a template for the next strand. Bases are matched to synthesize the new partner strands.<br /> DNA replication is a biological process that occurs in all living organisms and copies their DNA; it is the basis for biological inheritance. The process starts when one double-stranded DNA molecule produces two identical copies of the molecule. The cell cycle (mitosis) also pertains to the DNA replication/reproduction process. The cell cycle includes interphase, prophase, metaphase, anaphase, and telophase. Each strand of the original double-stranded DNA molecule serves as template for the production of the complementary strand, a process referred to as semiconservative replication. Cellular proofreading and error toe-checking mechanisms ensure near perfect fidelity for DNA replication.[1][2]<br /> In a cell, DNA replication begins at specific locations in the genome, called "origins".[3] Unwinding of DNA at the origin, and synthesis of new strands, forms a replication fork. In addition to DNA polymerase, the enzyme that synthesizes the new DNA by adding nucleotides matched to the template strand, a number of other proteins are associated with the fork and assist in the initiation and continuation of DNA synthesis.<br /> DNA replication can also be performed in vitro (artificially, outside a cell). DNA polymerases, isolated from cells, and artificial DNA primers are used to initiate DNA synthesis at known sequences in a template molecule. The polymerase chain reaction (PCR), a common laboratory technique, employs such artificial synthesis in a cyclic manner to amplify a specific target DNA fragment from a pool of DNA.<br /> Contents [hide]<br /> 1 DNA structure<br /> 2 DNA polymerase<br /> 3 Replication process<br /> 3.1 Origins<br /> 3.2 DNA replication proteins<br /> 3.3 Replication fork<br /> 3.3.1 Leading strand<br /> 3.3.2 Lagging strand<br /> 3.3.3 Dynamics at the replication fork<br /> 3.4 Regulation<br /> 3.5 Termination<br /> 4 Polymerase chain reaction<br /> 5 References<br /> [edit]DNA structure<br /> <br /> DNA usually exists as a double-stranded structure, with both strands coiled together to form the characteristic double-helix. Each single strand of DNA is a chain of four types of nucleotides having the bases: adenine, cytosine, guanine, and thymine (commonly noted as A,C, G & T). A nucleotide is a mono-, di-, or triphosphate deoxyribonucleoside; that is, a deoxyribose sugar is attached to one, two, or three phosphates, and a base. Chemical interaction of these nucleotides forms phosphodiester linkages, creating the phosphate-deoxyribose backbone of the DNA double helix with the bases pointing inward. Nucleotides (bases) are matched between strands through hydrogen bonds to form base pairs. Adenine pairs with thymine (two hydrogen bonds), and cytosine pairs with guanine (three hydrogen bonds) because a purine must pair with a pyrimidine: a purine cannot pair with another purine because the strands would be very close to each other; in a pyrimidine pair, the strands would be too far apart and the structure would be unstable. If A-C paired, there would be one hydrogen not bound to anything, making the DNA unstable.<br /> DNA strands have a directionality, and the different ends of a single strand are called the "3' (three-prime) end" and the "5' (five-prime) end" with the direction of the naming going 5 prime to the 3 prime region. The strands of the helix are anti-parallel with one being 5 prime to 3 then the opposite strand 3 prime to 5. These terms refer to the carbon atom in deoxyribose to which the next phosphate in the chain attaches. Directionality has consequences in DNA synthesis, because DNA polymerase can synthesize DNA in only one direction by adding nucleotides to the 3' end of a DNA strand.<br /> The pairing of bases in DNA through hydrogen bonding means that the information contained within each strand is redundant. The nucleotides on a single strand can be used to reconstruct nucleotides on a newly synthesized partner strand.[4]<br /> [edit]DNA polymerase<br /> <br /> Main article: DNA polymerase<br /> <br /> <br /> DNA polymerases adds nucleotides to the 3' end of a strand of DNA.[5] If a mismatch is accidentally incorporated, the polymerase is inhibited from further extension. Proofreading removes the mismatched nucleotide and extension continues.<br /> DNA polymerases are a family of enzymes that carry out all forms of DNA replication.[6] However, a DNA polymerase can only extend an existing DNA strand paired with a template strand; it cannot begin the synthesis of a new strand. To begin synthesis, a short fragment of DNA or RNA, called a primer, must be created and paired with the template DNA strand.<br /> DNA polymerase then synthesizes a new strand of DNA by extending the 3' end of an existing nucleotide chain, adding new nucleotides matched to the template strand one at a time via the creation of phosphodiester bonds. The energy for this process of DNA polymerization comes from two of the three total phosphates attached to each unincorporated base. (Free bases with their attached phosphate groups are called nucleoside triphosphates.) When a nucleotide is being added to a growing DNA strand, two of the phosphates are removed and the energy produced creates a phosphodiester bond that attaches the remaining phosphate to the growing chain. The energetics of this process also help explain the directionality of synthesis - if DNA were synthesized in the 3' to 5' direction, the energy for the process would come from the 5' end of the growing strand rather than from free nucleotides.<br /> In general, DNA polymerases are extremely accurate, making less than one mistake for every 107 nucleotides added.[7] Even so, some DNA polymerases also have proofreading ability; they can remove nucleotides from the end of a strand in order to correct mismatched bases. If the 5' nucleotide needs to be removed during proofreading, the triphosphate end is lost. Hence, the energy source that usually provides energy to add a new nucleotide is also lost.<br /> [edit]Replication process<br /> <br /> Main articles: Prokaryotic DNA replication and Eukaryotic DNA replication<br /> DNA Replication like all biological polymerization processes proceeds in three enzymatically catalyzed and coordinated steps. 1.INITIATION 2.ELONGATION 3.TERMINATION<br /> [edit]Origins<br /> For a cell to divide, it must first replicate its DNA.[8] This process is initiated at particular points in the DNA, known as "origins", which are targeted by proteins that separate the two strands and initiate DNA synthesis.[3] Origins contain DNA sequences recognized by replication initiator proteins (e.g., DnaA in E. coli' and the Origin Recognition Complex in yeast).[9] These initiators recruit other proteins to separate the strands and initiate replication forks.<br /> Initiator proteins recruit other proteins and form the pre-replication complex, which separate the DNA strands at the origin and forms a bubble. Origins tend to be "AT-rich" (rich in adenine and thymine bases) to assist this process, because A-T base pairs have two hydrogen bonds (rather than the three formed in a C-G pair)—in general, strands rich in these nucleotides are easier to separate because a greater number of hydrogen bonds requires more energy to break them.[10]<br /> All known DNA replication systems require a free 3' OH group before synthesis can be initiated (Important note: DNA is read in 3' to 5' direction whereas a new strand is synthesised in the 5' to 3' direction - this is entirely logical but is often confused). Four distinct mechanisms for synthesis have been described.<br /> 1. All cellular life forms and many DNA viruses, phages and plasmids use a primase to synthesize a short RNA primer with a free 3′ OH group which is subsequently elongated by a DNA polymerase.<br /> 2. The retroelements (including retroviruses) employ a transfer RNA that primes DNA replication by providing a free 3′ OH that is used for elongation by the reverse transcriptase.<br /> 3. In the adenoviruses and the φ29 family of bacteriophages, the 3' OH group is provided by the side chain of an amino acid of the genome attached protein (the terminal protein) to which nucleotides are added by the DNA polymerase to form a new strand.<br /> 4. In the single stranded DNA viruses - a group that includes the circoviruses, the geminiviruses, the parvoviruses and others - and also the many phages and plasmids that use the rolling circle replication (RCR) mechanism, the RCR endonuclease creates a nick the genome strand (single stranded viruses) or one of the DNA strands (plasmids). The 5′ end of the nicked strand is transferred to a tyrosine residue on the nuclease and the free 3′ OH group is then used by the DNA polymerase for new strand synthesis.<br /> The best known of these mechanisms is that used by the cellular organisms. In these once the two strands are separated, RNA primers are created on the template strands. To be more specific, the leading strand receives one RNA primer per active origin of replication while the lagging strand receives several; these several fragments of RNA primers found on the lagging strand of DNA are called Okazaki fragments, named after their discoverer. DNA polymerase extends the leading strand in one continuous motion and the lagging strand in a discontinuous motion (due to the Okazaki fragments). RNase removes the RNA fragments used to initiate replication by DNA polymerase, and another DNA Polymerase enters to fill the gaps. When this is complete, a single nick on the leading strand and several nicks on the lagging strand can be found. Ligase works to fill these nicks in, thus completing the newly replicated DNA molecule.<br /> The primase used in this process differs significantly between bacteria and archaea/eukaryotes. Bacteria use a primase belonging to the DnaG protein superfamily which contains a catalytic domain of the TOPRIM fold type. The TOPRIM fold contains an α/β core with four conserved strands in a Rossmann-like topology. This structure is also found in the catalytic domains of topoisomerase Ia, topoisomerase II, the OLD-family nucleases and DNA repair proteins related to the RecR protein.<br /> The primase used by archaea and eukaryotes in contrast contains a highly derived version of the RNA recognition motif (RRM). This primase is structurally similar to many viral RNA dependent RNA polymerases, reverse transcriptases, cyclic nucleotide generating cyclases and DNA polymerases of the A/B/Y families that are involved in DNA replication and repair. All these proteins share a catalytic mechanism of di-metal-ion-mediated nucleotide transfer, whereby two acidic residues located at the end of the first strand and between the second and third strands of the RRM-like unit respectively, chelate two divalent cations.<br /> As DNA synthesis continues, the original DNA strands continue to unwind on each side of the bubble, forming a replication fork with two prongs. In bacteria, which have a single origin of replication on their circular chromosome, this process eventually creates a "theta structure" (resembling the Greek letter theta: θ). In contrast, eukaryotes have longer linear chromosomes and initiate replication at multiple origins within these.[citation needed]<br /> [edit]DNA replication proteins<br /> List of major DNA replication enzymes in the Replisome:[11]<br /> Enzyme Function in DNA replication<br /> DNA Helicase Also known as helix destabilizing enzyme. Unwinds the DNA double helix at the Replication Fork.<br /> DNA Polymerase Builds a new duplex DNA strand by adding nucleotides in the 5' to 3' direction. Also performs proof-reading and error correction.<br /> DNA clamp A protein which prevents DNA polymerase III from dissociating from the DNA parent strand.<br /> Single-Strand Binding (SSB) Proteins Bind to ssDNA and prevent the DNA double helix from re-annealing after DNA helicase unwinds it thus maintaining the strand separation.<br /> Topoisomerase Relaxes the DNA from its super-coiled nature.<br /> DNA Gyrase Relieves strain of unwinding by DNA helicase.<br /> DNA Ligase Re-anneals the semi-conservative strands and joins Okazaki Fragments of the lagging strand.<br /> Primase Provides a starting point of RNA (or DNA) for DNA polymerase to begin synthesis of the new DNA strand.<br /> Telomerase Lengthens telomeric DNA by adding repetitive nucleotide sequences to the ends of eukaryotic chromosomes.<br /> [edit]Replication fork<br /> <br /> <br /> Scheme of the replication fork.<br /> a: template, b: leading strand, c: lagging strand, d: replication fork, e: primer, f: Okazaki fragments<br /> <br /> <br /> Many enzymes are involved in the DNA replication fork.<br /> The replication fork is a structure that forms within the nucleus during DNA replication. It is created by helicases, which break the hydrogen bonds holding the two DNA strands together. The resulting structure has two branching "prongs", each one made up of a single strand of DNA. These two strands serve as the template for the leading and lagging strands, which will be created as DNA polymerase matches complementary nucleotides to the templates; The templates may be properly referred to as the leading strand template and the lagging strand template.<br /> [edit]Leading strand<br /> The leading strand is the template strand of the DNA double helix so that the replication fork moves along it in the 3' to 5' direction. This allows the newly synthesized strand complementary to the original strand to be synthesized 5' to 3' in the same direction as the movement of the replication fork.<br /> On the leading strand, a polymerase "reads" the DNA and adds nucleotides to it continuously. This polymerase is DNA polymerase III (DNA Pol III) in prokaryotes and presumably Pol ε[7][12] in yeasts. In human cells the leading and lagging strands are synthesized by Pol α and Pol δ within the nucleus and Pol γ in the mitochondria. Pol ε can substitute for Pol δ in special circumstances.[13]<br /> [edit]Lagging strand<br /> The lagging strand is the strand of the template DNA double helix that is oriented so that the replication fork moves along it in a 5' to 3' manner. Because of its orientation, opposite to the working orientation of DNA polymerase III, which moves on a template in a 3' to 5' manner, replication of the lagging strand is more complicated than that of the leading strand.<br /> On the lagging strand, primase "reads" the DNA and adds RNA to it in short, separated segments. In eukaryotes, primase is intrinsic to Pol α.[14] DNA polymerase III or Pol δ lengthens the primed segments, forming Okazaki fragments. Primer removal in eukaryotes is also performed by Pol δ.[15] In prokaryotes, DNA polymerase I "reads" the fragments, removes the RNA using its flap endonuclease domain (RNA primers are removed by 5'-3' exonuclease activity of polymerase I [weaver, 2005], and replaces the RNA nucleotides with DNA nucleotides (this is necessary because RNA and DNA use slightly different kinds of nucleotides). DNA ligase joins the fragments together.<br /> [edit]Dynamics at the replication fork<br /> <br /> <br /> The assembled human DNA clamp, a trimer of the protein PCNA.<br /> As helicase unwinds DNA at the replication fork, the DNA ahead is forced to rotate. This process results in a build-up of twists in the DNA ahead.[16] This build-up would form a resistance that would eventually halt the progress of the replication fork. DNA Gyrase is an enzyme that temporarily breaks the strands of DNA, relieving the tension caused by unwinding the two strands of the DNA helix; DNA Gyrase achieves this by adding negative supercoils to the DNA helix.[17]<br /> Bare single-stranded DNA tends to fold back on itself and form secondary structures; these structures can interfere with the movement of DNA polymerase. To prevent this, single-strand binding proteins bind to the DNA until a second strand is synthesized, preventing secondary structure formation.[18]<br /> Clamp proteins form a sliding clamp around DNA, helping the DNA polymerase maintain contact with its template, thereby assisting with processivity. The inner face of the clamp enables DNA to be threaded through it. Once the polymerase reaches the end of the template or detects double-stranded DNA, the sliding clamp undergoes a conformational change that releases the DNA polymerase. Clamp-loading proteins are used to initially load the clamp, recognizing the junction between template and RNA primers.[citation needed]<br /> [edit]Regulation<br /> <br /> <br /> The cell cycle of eukaryotic cells.<br /> Eukaryotes<br /> Within eukaryotes, DNA replication is controlled within the context of the cell cycle. As the cell grows and divides, it progresses through stages in the cell cycle; DNA replication occurs during the S phase (synthesis phase). The progress of the eukaryotic cell through the cycle is controlled by cell cycle checkpoints. Progression through checkpoints is controlled through complex interactions between various proteins, including cyclins and cyclin-dependent kinases.[19]<br /> The G1/S checkpoint (or restriction checkpoint) regulates whether eukaryotic cells enter the process of DNA replication and subsequent division. Cells that do not proceed through this checkpoint remain in the G0 stage and do not replicate their DNA.<br /> Replication of chloroplast and mitochondrial genomes occurs independent of the cell cycle, through the process of D-loop replication.<br /> Bacteria<br /> Most bacteria do not go through a well-defined cell cycle but instead continuously copy their DNA; during rapid growth, this can result in the concurrent occurrences of multiple rounds of replication.[20] In E. coli, the best-characterized bacteria, DNA replication is regulated through several mechanisms, including: the hemimethylation and sequestering of the origin sequence, the ratio of ATP to ADP, and the levels of protein DnaA. All these control the process of initiator proteins binding to the origin sequences.<br /> Because E. coli methylates GATC DNA sequences, DNA synthesis results in hemimethylated sequences. This hemimethylated DNA is recognized by the protein SeqA, which binds and sequesters the origin sequence; in addition, DnaA (required for initiation of replication) binds less well to hemimethylated DNA. As a result, newly replicated origins are prevented from immediately initiating another round of DNA replication.[21]<br /> ATP builds up when the cell is in a rich medium, triggering DNA replication once the cell has reached a specific size. ATP competes with ADP to bind to DnaA, and the DnaA-ATP complex is able to initiate replication. A certain number of DnaA proteins are also required for DNA replication — each time the origin is copied, the number of binding sites for DnaA doubles, requiring the synthesis of more DnaA to enable another initiation of replication.<br /> [edit]Termination<br /> Eukaryotes initiate DNA replication at multiple points in the chromosome, so replication forks meet and terminate at many points in the chromosome; these are not known to be regulated in any particular way. Because eukaryotes have linear chromosomes, DNA replication is unable to reach the very end of the chromosomes, but ends at the telomere region of repetitive DNA close to the end. This shortens the telomere of the daughter DNA strand. This is a normal process in somatic cells. As a result, cells can only divide a certain number of times before the DNA loss prevents further division. (This is known as the Hayflick limit.) Within the germ cell line, which passes DNA to the next generation, telomerase extends the repetitive sequences of the telomere region to prevent degradation. Telomerase can become mistakenly active in somatic cells, sometimes leading to cancer formation.<br /> Because bacteria have circular chromosomes, termination of replication occurs when the two replication forks meet each other on the opposite end of the parental chromosome. E coli regulate this process through the use of termination sequences that, when bound by the Tus protein, enable only one direction of replication fork to pass through. As a result, the replication forks are constrained to always meet within the termination region of the chromosome.[22]<br /> [edit]Polymerase chain reaction<br /> <br /> Main article: Polymerase chain reaction<br /> Researchers commonly replicate DNA in vitro using the polymerase chain reaction (PCR). PCR uses a pair of primers to span a target region in template DNA, and then polymerizes partner strands in each direction from these primers using a thermostable DNA polymerase. Repeating this process through multiple cycles produces amplification of the targeted DNA region. At the start of each cycle, the mixture of template and primers is heated, separating the newly synthesized molecule and template. Then, as the mixture cools, both of these become templates for annealing of new primers, and the polymerase extends from these. As a result, the number of copies of the target region doubles each round, increasing exponentially.[23]

LHMC-Lady Hardinge Medical College
Promotion Mix notes for Lady H ...
<p style="margin-top: 15px; margin-right: 0px; margin-bottom: 15px; margin-left: 0px; padding-top: 0px; padding-right: 0px; padding-bottom: 0px; padding-left: 0px; border-top-width: 0px; border-right-width: 0px; border-bottom-width: 0px; border-left-width: 0px; border-style: initial; border-color: initial; outline-width: 0px; outline-style: initial; outline-color: initial; font-size: 14px; background-image: initial; background-attachment: initial; background-origin: initial; background-clip: initial; background-color: rgb(255, 255, 255); font-family: Georgia, Cambria, 'Times New Roman', Times, serif; line-height: 20px; text-align: justify; "> Promotion is an important part of marketing mix of a business enterprise. Once a product is developed, its price is determined the next problem comes to its sale i.e., creating demand for the product. It requires promotional activities. The activities are technique which bring the special characteristics of the product and of the producer to the knowledge of prospective customers. Promotion is a process of communication involving information, persuasion, and influence. The term 'selling' is often used synonymously with promotion. But promotion is wider that selling. Selling is concerned only with the transfer of title in goods to the purchaser, whereas promotion includes techniques stimulating demand. These techniques include advertising, salesmanship or personal selling and other methods of stimulation demand.<br /> Advertising and sales promotion techniques are indirect and non-personal whereas personal selling or salesmanship is a direct and personal technique. All these techniques, however, should be integrated with the marketing objective of the enterprise. The salesmen can report about the different advertising and other promotional appeals as they are in close touch with the consumer public and market conditions.<br /> Promotion is essentially the sales efforts of a business enterprise and includes the function of informing, persuading and influencing the purchase decision of the existing the prospective consumers with the object of increasing sales volume and profits. Promotion is the efforts of the seller to sell the product effectively. Promotion is the communication with the customers to pursue them to buy the product. It is the duty of the marketing manager to choose the communication media and blend them into an effective promotion programme. These are more than one type of tools used to promote sales. The combination of these tools with a view to maintain and create sales is known as promotion mix. Promotion mix is the name given to the combination of methods used in communicating with customers. There are four tools of promotion mix viz. advertisement, personal selling, publicity and sales promotion. These are called elements of promotion mix.<br /> <br /> Elements of Promotion Mix<br /> <br /> <strong>There are four elements of promotion mix:</strong><br /> <br /> <strong>Advertising</strong><br /> Advertising is a non-personal presentation of goods, services or idea. In advertising existing and prospective customers are communicated the message through impersonal media like radio, T.V., newspapers and magazine. It involves transmission of standard message simultaneously to a large number of people. The message transmitted is known as advertising.<br /> <br /> <strong>Personal Selling</strong><br /> Personal selling is the process of assisting and persuading the existing and prospective buyer to buy the goods or services in person. It involves direct and personal contact of the seller or his representative with the buyer.<br /> <br /> <strong>Publicity</strong><br /> Publicity is a non-personal non-paid stimulation of demand of the product or services or business unit by planning commercially significant news about the services or business unit by planning commercially significant news about in the print media or by obtaining a favorable presentation of it upon radio, television or stage.<br /> <br /> <strong>Sales promotion</strong><br /> Sales promotion consists of all activities other than advertising, personal selling and publicity, which help in promoting sales of the product. Such activities are non-repetitive and one time offers. According to American Marketing Association, sales promotion include, "those marketing activities other than personal selling, advertising and publicity that stimulate consumer purchasing and dealer effectiveness, such as point of purchase displays, shows and exhibitions, demonstrations and various non-recurring selling efforts not in the ordinary routine."<br /> The main aim of sales promotion is to increase sales and profits of the firm but it is quite different from personal selling and advertising. In personal selling, customer is persuaded by a sales person face to face. Advertising is a non-personal mass communication media. Sales promotion, on the other hand, is a non-recurring and non-routine method. Its main aim is to supplement and coordinate the personal selling and advertising. It is a supporting and facilitating element of promotional strategy. Sales promotion bridges the gap of advertising and personal selling.</p>

ICFAI-ICFAI University
Money Banking and Credit Manag ...
Money, Banking and Credit Management is the paper of the MBA Degree under the discipline of management studies from the ICFAI University.The complete questions are arranged in three different sections A, B and C and full marks allotted for this paper is 100.Every section in this paper has different types of questions. The first section deals with multiple types and the second part includes short answer type. The last section contains descriptive type questions.<br />

ACACS-Abhinav College of Arts Commerce and Science
MBA 1st year Research Methods ...
Research Methods for Management is the paper of MBA, 1st year in Bharathiar university.The paper consists of a total of 100 marks and the time allotted for its completion is 3 hours. The paper consist of 8 questions out of which 5 must be answered. The paper may or may not have sub-parts.

JNC-Jyoti Nivas College
politics 1 notes for student o ...
Indian Railways also pay’s a large amount of its revenue to Indian Army for there salaries and basic needs<br /> Helping tourism sector to become more efficient and profit earning.

JNC-Jyoti Nivas College
politics 2 notes for student o ...
<strong>Russian federation</strong><br /> From 1922 until December 25, 1991, the Russian Federation formed part of the Union of Soviet Socialist Republics (USSR; or Soviet Union). <br /> In the year 1922, Russian Federation became one of the USSR’s 15 constituent republics— the largest and most influential, accounting for more than three quarters of its area and more than half of its population.
MH-Miranda House
genomic RNA isolation
RNA Isolation Protocol<br /> (Revised 5-15-2003)<br /> <br /> Stabilize RNA<br /> <br /> Start with 15 ml E. coli Culture containing 7.5* 109 cells (OD600= 0.2 Dilute cells or scale up)<br /> Pipet 30 ml of RNAProtect Bacteria Reagent (Qiagen) into a 50ml polypropylene conical tube.<br /> Pipet 15 ml culture into the tube. Mix immediately by vortexing for 5second. Incubate for 5min at room temperature.<br /> Centrifuge for 10min at 5800g( 4500rpm for H-6000A rotor in SORVALL RC-3B centrifuge)<br /> Decant the supernatant, and leave tubes inverted on a paper towel for 10s.<br /> Freeze the pellet with EtOH/Dye Ice mix.<br /> The pellet can be stored at -20°C up to 2 weeks, or -70°C for up to 4 weeks.<br /> Isolation RNA<br /> <br /> Dilute 2ul of Proteinase K into 300ul of Tissue and Cell Lysis solution for each sample.<br /> Resuspend cell pellet by the Lysis solution and mix thoroughly. Transfer mix to 1.5ml tube.<br /> Incubate at 65°C for 45min, and vortex every 15min.<br /> Place the sample on ice for 5min.<br /> Add 150ul of MPC protein Precipitation Reagent to 300ul of lysed sample and vortex mix for 10sec.<br /> Spin for 10min 4°C at max speed in a microcentrifuge. Transfer the supernant to a clean tube.<br /> Add 50ul of MPC protein Precipitation Reagent and repeat above step.<br /> Add 500ul isopropanol to the recovered supernatant, invert the tube 30-40 times.<br /> Pellet the RNA by centrifugation at 4°C for 10min in a microcentrifuge.<br /> Carefully pour off the isopropanol without dislodging the RNA pellet. Remove all of the residual isopropanol with a pipet. Air dry 10-15min.<br /> Removal of contaminating DNA<br /> <br /> Dilute 10ul of RNAse-Free DNAse I up to 200ul with 1x DNAse Buffer for each sample.<br /> Completely resuspend the nucleic acid pellet in 200ul of DNAse I solution.<br /> Incubation at 37deg;C for 45min<br /> Add 200ul of 2x T and C lysis solution, vortex mix for 5 seconds<br /> Add 200ul of MPC reagent vortex mix 10seconds, place on ice 5min.<br /> Pellet the debris by centrifugation for 10min at 4°C, >10,000g in a microcentrifuge.<br /> Add 50ul of MPC reagent and repeat 5 and 6 (but this time spin 20min).<br /> Add 600ul isopropanol to the recovered supernatant, invert the tube 30-40 times.<br /> Pellet the RNA by centrifugation at 4°C for 10min in a microcentrifuge.<br /> Carefully pour off the isopropanol without dislodging the RNA pellet.<br /> Rinse with 75% EtOH (DEPC H2O), being careful to not dislodge the pellet. Centrifuge 5min at 4°C<br /> Remove all of the residual EtOH with a pipet. Air dry 10-15min.<br /> Resuspend the nucleic acid in 52ul RNAse-free water.<br /> Storage, Quantitation and Determination of Quality of RNA<br /> <br /> Electrophoresis on 1% Agarose gel with 1ul sample.<br /> Dilute 1ul sample to 100ul with TE(10mM Tris.HCl pH 8, 1mM EDTA), and measure A260 and A280 with a spectrophotometer.. Alternatively 1ul sample can be used to measure A260 and A280 on a Nanodrop ND-1000 Spectrophotometer without dilution.<br /> Concentration of RNA sample = 40 x A260 x 100 (Dilution factor) (ug/ml)<br /> A260/A280 Ratio = A260/A280, ranging from 1.7 to 2.1<br /> Add 100ul EtOH (2 volume) and 5ul 3M NaOAC (1/10 volume), store at -20°C<br /> Regents<br /> <br /> RNAProtect Bacteria Reagent Qiagen<br /> MasterPure RNA Isolation Kit Epicentre<br />
MH-Miranda House
genomic RNA isolation
RNA Isolation Protocol<br /> (Revised 5-15-2003)<br /> <br /> Stabilize RNA<br /> <br /> Start with 15 ml E. coli Culture containing 7.5* 109 cells (OD600= 0.2 Dilute cells or scale up)<br /> Pipet 30 ml of RNAProtect Bacteria Reagent (Qiagen) into a 50ml polypropylene conical tube.<br /> Pipet 15 ml culture into the tube. Mix immediately by vortexing for 5second. Incubate for 5min at room temperature.<br /> Centrifuge for 10min at 5800g( 4500rpm for H-6000A rotor in SORVALL RC-3B centrifuge)<br /> Decant the supernatant, and leave tubes inverted on a paper towel for 10s.<br /> Freeze the pellet with EtOH/Dye Ice mix.<br /> The pellet can be stored at -20°C up to 2 weeks, or -70°C for up to 4 weeks.<br /> Isolation RNA<br /> <br /> Dilute 2ul of Proteinase K into 300ul of Tissue and Cell Lysis solution for each sample.<br /> Resuspend cell pellet by the Lysis solution and mix thoroughly. Transfer mix to 1.5ml tube.<br /> Incubate at 65°C for 45min, and vortex every 15min.<br /> Place the sample on ice for 5min.<br /> Add 150ul of MPC protein Precipitation Reagent to 300ul of lysed sample and vortex mix for 10sec.<br /> Spin for 10min 4°C at max speed in a microcentrifuge. Transfer the supernant to a clean tube.<br /> Add 50ul of MPC protein Precipitation Reagent and repeat above step.<br /> Add 500ul isopropanol to the recovered supernatant, invert the tube 30-40 times.<br /> Pellet the RNA by centrifugation at 4°C for 10min in a microcentrifuge.<br /> Carefully pour off the isopropanol without dislodging the RNA pellet. Remove all of the residual isopropanol with a pipet. Air dry 10-15min.<br /> Removal of contaminating DNA<br /> <br /> Dilute 10ul of RNAse-Free DNAse I up to 200ul with 1x DNAse Buffer for each sample.<br /> Completely resuspend the nucleic acid pellet in 200ul of DNAse I solution.<br /> Incubation at 37deg;C for 45min<br /> Add 200ul of 2x T and C lysis solution, vortex mix for 5 seconds<br /> Add 200ul of MPC reagent vortex mix 10seconds, place on ice 5min.<br /> Pellet the debris by centrifugation for 10min at 4°C, >10,000g in a microcentrifuge.<br /> Add 50ul of MPC reagent and repeat 5 and 6 (but this time spin 20min).<br /> Add 600ul isopropanol to the recovered supernatant, invert the tube 30-40 times.<br /> Pellet the RNA by centrifugation at 4°C for 10min in a microcentrifuge.<br /> Carefully pour off the isopropanol without dislodging the RNA pellet.<br /> Rinse with 75% EtOH (DEPC H2O), being careful to not dislodge the pellet. Centrifuge 5min at 4°C<br /> Remove all of the residual EtOH with a pipet. Air dry 10-15min.<br /> Resuspend the nucleic acid in 52ul RNAse-free water.<br /> Storage, Quantitation and Determination of Quality of RNA<br /> <br /> Electrophoresis on 1% Agarose gel with 1ul sample.<br /> Dilute 1ul sample to 100ul with TE(10mM Tris.HCl pH 8, 1mM EDTA), and measure A260 and A280 with a spectrophotometer.. Alternatively 1ul sample can be used to measure A260 and A280 on a Nanodrop ND-1000 Spectrophotometer without dilution.<br /> Concentration of RNA sample = 40 x A260 x 100 (Dilution factor) (ug/ml)<br /> A260/A280 Ratio = A260/A280, ranging from 1.7 to 2.1<br /> Add 100ul EtOH (2 volume) and 5ul 3M NaOAC (1/10 volume), store at -20°C<br /> Regents<br /> <br /> RNAProtect Bacteria Reagent Qiagen<br /> MasterPure RNA Isolation Kit Epicentre<br />

ICFAI-ICFAI University
Business Ethics and Corporate ...
Business Ethics and Corporate Governance is the paper of the MBA Degree under the discipline of business management from the ICFAI University.The total mark is 80 and the candidate has to complete the paper in 2 and ½ hours. 30 minutes is meant for answering section A and the rest is meant for section B.<br />

AMSSOI-Andhra Mahila Sabha School of Informatics
International Business notes of Osmania University
<strong>Introduction:</strong><br /> International business (IB) means business or commercial transactions that take place between two or more than two countries. It can be conducted in many ways like import- export of goods and services, issuing license, international franchising to produce goods in other country, foreign direct investment (FDI), providing outsource services to the other companies across the nation, starting joint venture with a company etc.<br /> <br /> <strong>Role of IB in Economic development:</strong><br /> Since 1980’s there is a high competition at global level. International business has a great scope in private sector. The operations are conducted on large scale and hence it also provides wide area of job opportunities which helps in sound employment ratio. The developing countries get an opportunity to get advance technology and foreign capital which helps in industrial development and leads to overall economic development.<br /> <br /> Government sector participate in international business for strong political relations.<br /> <br /> <strong>Challenges:</strong><br /> International business goes through the number of economic, political and other business formalities. So, it gets affected by any change in economic and political conditions. Hence it is very sensitive in nature. To overcome from this sensitive nature and to achieve business goals in Competitive market, all MNC’s or International business plans some strategies.<br /> <br /> *Note: Strategies is an approach developed by an organization to achieve its particular business objectives. It is different from policies. Policies guide the plan of action and decisions.<br /> <br /> <strong>International business strategy</strong>:<br /> <br /> It is a planned action that governs commercial activities conducted between multiple countries to achieve certain set of goals. International business is similar to national business, but there are some differences which have to be consider for successful execution of international strategies.<br /> <br /> Some of the key areas and strategies of international business are: Business entry strategies like exporting, franchising, licensing, joint venture, tax related; political strategies, Humanities promotion & marketing strategies, human resource management (HRM marketing) strategy, product and resource related strategies, cost and profit related etc.
AU-Amity University
Attitude plays an important role
<span style="color:rgb(37, 37, 37)">An </span><strong>attitude</strong><span style="color:rgb(37, 37, 37)"> is an expression of favor or disfavor toward a person, place, thing, or event. It is one of</span><span style="color:rgb(37, 37, 37)"> "the most distinctive and indispensable concept in contemporary.</span><span style="color:rgb(37, 37, 37)">Attitude can be formed from a person's past and present.</span><span style="color:rgb(37, 37, 37)">Attitude is also measurable and changeable as well as influencing the person's emotion and behavior.</span>
AU-Amity University
Attitude plays an important role
<span style="color:rgb(37, 37, 37)">An </span><strong>attitude</strong><span style="color:rgb(37, 37, 37)"> is an expression of favor or disfavor toward a person, place, thing, or event. It is one of</span><span style="color:rgb(37, 37, 37)"> "the most distinctive and indispensable concept in contemporary.</span><span style="color:rgb(37, 37, 37)">Attitude can be formed from a person's past and present.</span><span style="color:rgb(37, 37, 37)">Attitude is also measurable and changeable as well as influencing the person's emotion and behavior.</span>
MH-Miranda House
genimic DNA isolation
2/19/2010<br /> 1 of 6<br /> Isolation and Purification of Total Genomic DNA from E. coli<br /> INTRODUCTION<br /> The isolation and purification of DNA from cells is one of the most common procedures in contemporary<br /> molecular biology and embodies a transition from cell biology to the molecular biology; from in vivo to<br /> in vitro, if you prefer.<br /> DNA was first isolated as long ago as 1869 by Friedrich Miescher while he was a postdoctoral student at<br /> the University of Tübingen. Miesher obtained his first DNA, which he referred to it as nuclein, from<br /> human leukocytes washed from pus-laden bandages amply supplied by surgical clinics in the time<br /> before antibiotics. He continued to study DNA as a professor at the University of Basel, but switched<br /> from leukocytes to salmon sperm as his starting material. Meisher’s choice of starting material was<br /> based on the knowledge that leukocytes and sperm have large nuclei relative to cell size. DNA isolated<br /> from salmon sperm and from (bovine) lymphocytes is still available commercially.<br /> Molecular biologists distinguish genomic DNA isolation from plasmid DNA isolation. If you have taken<br /> the Biology 20L class you have done plasmid DNA isolation from E. coli using a procedure based on a<br /> commercial kit from Promega (“Wizard Miniprep” System). Plasmid DNA isolation is more demanding<br /> than genomic DNA isolation because plasmid DNA must be separated from chromosomal DNA,<br /> whereas a genomic DNA isolation needs only to separate total DNA from RNA, protein, lipid, etc.<br /> Many different methods are available for isolating genomic DNA, and a number of biotech companies<br /> sell reagent kits. Choosing the most appropriate method for a specific application demands<br /> consideration of the issues below. No single method addresses all these issues to complete satisfaction.<br /> • SOURCE: What organism/tissue will the DNA come from?<br /> Some organisms present special difficulties for DNA isolation. Plants cells, for example, are<br /> considerably more difficult than animal cells, because of their cell walls, and require special<br /> attention.<br /> Most lab strains of E. coli are fairly straightforward, but a few E. coli strains produce high molecular<br /> weight polysaccharides that co-purify with DNA. You need to look into the genotype of the E. coli<br /> strain to know whether you need special steps to eliminate this extraneous material. Inasmuch as<br /> our E. coli isolates are direct from nature, we are relying on our procedure to deal with this issue.<br /> • YIELD: How much DNA do you need?<br /> If the source is limited, you will need to use a method that is very efficient at producing a<br /> high yield. Fortunately, E. coli is easy to grow, and PCR is effective with very small amounts of DNA<br /> sample, so this will not be a major issue for us.<br /> • PURITY: What level of contaminants (protein, RNA, etc.) can be tolerated?<br /> The purification method must eliminate any contaminants that would interfere with<br /> subsequent steps. This depends, of course, on what you plan to do with the DNA once you have<br /> isolated it. PCR will tolerate a reasonable degree of contamination so long as the contaminants do<br /> not inhibit the thermostable DNA polymerase or degrade DNA. We also need to strip proteins off the<br /> DNA so that it is a good template for replication.<br /> 2/19/2010<br /> 2 of 6<br /> • INTEGRITY: How large are the DNA fragments in our genomic preps?<br /> HMW DNA is notoriously fragile. It is easily cut into smaller pieces by hydrodynamic shearing forces and<br /> by DNases.<br /> Hydrodynamic shear is minimized by avoiding vigorous vortexing and pipetting of DNA solutions. A<br /> simple precaution is to use micropipette tips with orifices larger than usual (“wide bore tips).<br /> The DNases liberated from the lysed cells are usually inactivated by the protein denaturation step in the<br /> procedure. Occasionally DNases are introduced to the procedure as accidental contaminants of other<br /> reagents, particularly RNase. Many investigators buy special "Molecular Biology" grade reagents that<br /> have been certified "DNase-free" by the manufacturer. These are expensive. DNases present as<br /> contaminants in RNase solutions can be inactivated by boiling the RNase for 15 minutes.<br /> • ECONOMY: How much time and expense are involved?<br /> For example, CsCI density-gradient ultracentrifugation provides highly pure DNA samples in relatively<br /> high yield, and was formerly widely used. However, ultracentrifugation is very expensive because it<br /> requires an instrument costing around $ 40,000. Additionally, it is inconvenient because the<br /> centrifugation runs typically go many hours. So this method is now used mostly in situations where high<br /> yield and high purity are critical.<br /> Many biotech companies sell kits with all the reagents necessary for genomic preps. You<br /> need to look carefully at the cost of these kits relative to the labor that they save.<br /> • SAFETY<br /> Inasmuch as DNA isolation methods are designed to break cells and denature proteins, it is not<br /> surprising that some reasonably nasty reagents are involved.<br /> A phenol/chloroform reagent widely used in DNA purification is notoriously hazardous. In fact,<br /> phenol/chloroform is probably the most hazardous reagent used regularly in molecular biology labs.<br /> Phenol is a very strong acid that causes severe burns. Chloroform is a<br /> carcinogen. So, phenol/chloroform is a double whammy. It is not only dangerous, but<br /> expensive, when you consider the cost of hazardous waste disposal. Our procedure does not use<br /> phenol/chloroform.<br /> • LYSIS<br /> Cell walls and membranes must be broken to release the DNA and other intracellular components. This<br /> is usually accomplished with an appropriate combination of enzymes to digest the cell wall (usually<br /> lysozyme) and detergents to disrupt membranes. We use the ionic detergent Sodium Dodecyl Sulfate<br /> (SDS) at 80°C to lyse E. coli.<br /> • REMOVAL OF PROTEIN, CARBOHYDRATE, RNA ETC.<br /> RNA is usually degraded by the addition of RNase. The resulting oligoribinucleotides are separated from<br /> the high molecular weight (HMW) DNA by exploiting their differential solubilities in non-polar solvents<br /> (usually alcohol/water).<br /> Proteins are subjected to chemical denaturation and/or enzymatic degradadtion. The most common<br /> technique of protein removal involves denaturation and extraction into an organic phase consisting of<br /> phenol and chloroform.<br /> Another widely used purification technique is to band the DNA in a CsCl density gradient using<br /> ultracentrifugation.<br /> 2/19/2010<br /> 3 of 6<br /> Procedure<br /> WEAR GOGGLES AND GLOVES<br /> DISCARD REAGENTS AND TUBES IN LABELED WASTE CONTAINERS<br /> 1. Briefly vortex the overnight culture to ensure that cells are uniformly suspended. Then transfer<br /> 1.0 ml of the overnight culture to a 1.5ml microcentrifuge tube.<br /> 2. Centrifuge at 15,000 g (or max. speed) for 2 minutes to pellet the cells.<br /> Place your tubes opposite each other to balance to rotor. Do not initiate a spin cycle until the<br /> rotor is fully loaded; this minimizes the total number of runs required.<br /> A cell pellet should be visible at the bottom of the tube.<br /> 3. Transfer the supernatant back into the culture tube it came from and discard this culture tube<br /> as biohazard waste.<br /> Carefully remove as much of the supernatant as you can without disturbing the cell pellet. The<br /> pellet may be on the side of the tube, not squarely on the bottom. I use my P1000 set to 950<br /> ul.<br /> 4. Resuspend the cell pellet in 600μl of Lysis Solution (LS).<br /> Gently pipet until the cells are thoroughly resuspended and no cell clumps remain.<br /> LS contains the anionic detergent sodium dodecyl sulphate (SDS) to disrupt membranes and<br /> denature proteins. You may notice that the cell suspension is not as turbid as the cell culture<br /> you started with; this is because some cell lysis has already occurred.<br /> 5. Incubate at 80°C for 5 minutes to completely lyse the cells.<br /> The samples should now look clear.<br /> 6. Cool the tube contents to room temperature.<br /> Do not rely on temperature equilibration with ambient air. Place the tube in a room<br /> temperature water bath for several minutes.<br /> 7. Add 3μl of RNase solution to the cell lysate. Invert the tube 2–5 times to mix.<br /> 8. Incubate at 37°C for 30 minutes to digest RNA. Cool the sample to room temperature.<br /> This step is intended to degrade RNA into small fragments or individual ribonucleotides.<br /> 9. Add 200 μl of Protein Precipitation Solution (PPS) to the RNase-treated cell lysate.<br /> Vortex vigorously at high speed for 20 seconds. Do not skimp on the vortexing<br /> 2/19/2010<br /> 4 of 6<br /> 10. Incubate the sample in an ice/water slurry for 5 minutes.<br /> The sample now has significant whitish insoluble material.<br /> 11. Centrifuge at 15,000 g (or max. speed) for 3 minutes.<br /> There should be a large pellet of whitish gunk on the bottom and sides of the tube. The gunk<br /> consists of denatured proteins and fragments of membrane and cell wall.<br /> 12. Transfer the supernatant (≤800 μl) containing the DNA to a clean 1.5ml microcentrifuge tube<br /> containing 600μl of room temperature isopropanol (IPA).<br /> Be sure that you don’t suck up and transfer any of the grungy precipitate. I use my P1000 set<br /> to 750 ul.<br /> 13. Mix the DNA solution with the IPA by inverting the tube at least 15 times.<br /> The DNA is usually (barely) visible as a small floc of whitish material.<br /> 14. Centrifuge at 15,000 g (or max. speed) for 2 minutes.<br /> 15. Carefully pour off the supernatant (do not pipette) and invert the tube on clean absorbent<br /> paper to drain for 2-5 minutes. You want the paper to wick off the IPA that drains down and<br /> collects at the rim of the inverted tube.<br /> The DNA pellet may or may not be visible.<br /> Do not allow the DNA pellet to completely dry.<br /> 16. Add 600μl of room temperature 70% ethanol and gently invert the tube several times to wash<br /> the DNA pellet.<br /> Do not resuspend by pipetting.<br /> 17. Centrifuge at 15,000 g (or max. speed) for 2 minutes.<br /> 18. Carefully pour off the ethanol supernatant (do not pipette) and invert the tube on clean<br /> absorbent paper to drain. You want the paper to wick off the ethanol that drains down and<br /> collects at the rim of the inverted tube<br /> 19. Allow the pellet to air-dry for 10–15 minutes.<br /> You want to evaporate as much of the ethanol as possible without letting the DNA pellet<br /> completely dry. When all the EtOH is gone there should still be some water left hydrating the<br /> DNA.<br /> 20. Add 100μl of DNA Rehydration Solution (RH) to the tube and rehydrate the DNA by incubating<br /> at 65°C for 1 hour.<br /> After 30 minutes, flick the bottom of the tube gently to facilitate dissolution and mixing.<br /> 2/19/2010<br /> 5 of 6<br /> Alternatively, rehydrate the DNA by incubating the solution overnight at room temperature or<br /> at 4°C, preferably on a low speed shaker.<br /> 21. Analyze the DNA prep. by spectrophotometry using the Nanodrop spectrophotometer and then<br /> store the DNA at 2–8°C.<br /> Deteremine the A260 value and the A260/A280 ratio for your prep.<br /> Evaluating Yield, Purity and Size of the DNA in a Genomic Prep<br /> Before proceeding further into costly and time-consuming manipulations it is critical to analyze, at<br /> least in a cursory way, the quantity and quality of DNA in the prep.<br /> Estimating DNA Concentration by A260<br /> The UV absorbance spectrum of DNA exhibits an Amax @ 260 nm based on the aromatic ring<br /> structures of the DNA bases. This is the most convenient way to estimate DNA concentration and<br /> calculate yield, as long as the DNA preparation is relatively free of contaminants that absorb in the<br /> UV. Proteins, and residual phenol left from the isolation procedure, are typical contaminants that<br /> may lead to an overestimate DNA concentration.<br /> An A260 = 1.0 indicates a [DNA] = 50 ug/ul, assuming the DNA is pure.<br /> Detecting protein contamination by A260/A280<br /> Protein contaminants in a DNA prep also will absorb UV but their Amax = 280 nm. Therefore, the<br /> A260/A280 ratio can reveal the presence of gross amounts of protein contamination. Pure DNA has<br /> an A260/A280 ratio of 1.8-1.9. Lower ratios indicate substantial protein contamination. A higher<br /> ratio generally indicates RNA contamination.<br /> Agarose Gel Electrophoresis<br /> Gel electrophoresis can confirm that the DNA in your prep is HMW and will reveal if there is a<br /> large amount of RNA contamination. We will analyze the genomic DNA prep and several other<br /> samples by gel electrophoresis in a later lab.<br /> 2/19/2010<br /> 6 of 6<br /> Assignment<br /> 1. Make a rough calculation of the theoretical DNA yield in (in ug) and DNA concentration (in<br /> ng/ul) assuming that you recover 100% of the genomic DNA in pure form. You will need the<br /> following ballpark assumptions:<br /> 2 X 109 cells/ml Typical concentration of an overnight culture:<br /> 4,500 kb Approximate Genome size<br /> 616 g/mole Average MW of DNA base pair<br /> 1 ml Original culture volume<br /> 0.1 ml Final DNA sample volume<br /> 6.2 X 1023 molecules/mole Avogadro's #<br /> 2. Record the actual results of the analysis in the following format:<br /> 1 ml culture<br /> 2X109 cells/ml approx.<br /> vol. = ____ ul<br /> conc. = _____ ng/ul<br /> amount = _____ ug<br /> yield = _____%<br /> Genomic<br /> DNA Prep.<br /> "yield" is the % of theoretical yield calculated in part 1.<br /> 3. Briefly comment on the quality of your DNA prep.
MH-Miranda House
bioremediation general info
Bioremediation is the use of micro-organism metabolism to remove pollutants. Technologies can be generally classified as in situ or ex situ. In situ bioremediation involves treating the contaminated material at the site, while ex situ involves the removal of the contaminated material to be treated elsewhere. Some examples of bioremediation technologies are phytoremediation, bioventing, bioleaching, landfarming, bioreactor, composting, bioaugmentation, rhizofiltration, and biostimulation.<br /> Bioremediation can occur on its own (natural attenuation or intrinsic bioremediation) or can be spurred on via the addition of fertilizers to increase the bioavailability within the medium (biostimulation). Recent advancements have also proven successful via the addition of matched microbe strains to the medium to enhance the resident microbe population's ability to break down contaminants. Microorganisms used to perform the function of bioremediation are known as bioremediators.[1]<br /> Not all contaminants, however, are easily treated by bioremediation using microorganisms. For example, heavy metals such as cadmium and lead are not readily absorbed or captured by microorganisms. The assimilation of metals such as mercury into the food chain may worsen matters. Phytoremediation is useful in these circumstances because natural plants or transgenic plants are able to bioaccumulate these toxins in their above-ground parts, which are then harvested for removal.[2] The heavy metals in the harvested biomass may be further concentrated by incineration or even recycled for industrial use.<br /> The elimination of a wide range of pollutants and wastes from the environment requires increasing our understanding of the relative importance of different pathways and regulatory networks to carbon flux in particular environments and for particular compounds, and they will certainly accelerate the development of bioremediation technologies and biotransformation processes.[3]<br /> Contents [hide]<br /> 1 Genetic engineering approaches<br /> 2 Mycoremediation<br /> 3 Advantages<br /> 4 Monitoring bioremediation<br /> 5 See also<br /> 6 References<br /> 7 External links<br /> [edit]Genetic engineering approaches<br /> <br /> The use of genetic engineering to create organisms specifically designed for bioremediation has great potential.[4] The bacterium Deinococcus radiodurans (the most radioresistant organism known) has been modified to consume and digest toluene and ionic mercury from highly radioactive nuclear waste.[5]<br /> [edit]Mycoremediation<br /> <br /> Mycoremediation is a form of bioremediation in which fungi are used to decontaminate the area. The term mycoremediation refers specifically to the use of fungal mycelia in bioremediation.<br /> One of the primary roles of fungi in the ecosystem is decomposition, which is performed by the mycelium. The mycelium secretes extracellular enzymes and acids that break down lignin and cellulose, the two main building blocks of plant fiber. These are organic compounds composed of long chains of carbon and hydrogen, structurally similar to many organic pollutants. The key to mycoremediation is determining the right fungal species to target a specific pollutant. Certain strains have been reported to successfully degrade the nerve gases VX and sarin.<br /> In one conducted experiment, a plot of soil contaminated with diesel oil was inoculated with mycelia of oyster mushrooms; traditional bioremediation techniques (bacteria) were used on control plots. After four weeks, more than 95% of many of the PAH (polycyclic aromatic hydrocarbons) had been reduced to non-toxic components in the mycelial-inoculated plots. It appears that the natural microbial community participates with the fungi to break down contaminants, eventually into carbon dioxide and water. Wood-degrading fungi are particularly effective in breaking down aromatic pollutants (toxic components of petroleum), as well as chlorinated compounds (certain persistent pesticides; Battelle, 2000).<br /> Mycofiltration is a similar process, using fungal mycelia to filter toxic waste and microorganisms from water in soil.<br /> [edit]Advantages<br /> <br /> There are a number of cost/efficiency advantages to bioremediation, which can be employed in areas that are inaccessible without excavation. For example, hydrocarbon spills (specifically, petrol spills) or certain chlorinated solvents may contaminate groundwater, and introducing the appropriate electron acceptor or electron donor amendment, as appropriate, may significantly reduce contaminant concentrations after a long time allowing for acclimation. This is typically much less expensive than excavation followed by disposal elsewhere, incineration or other ex situ treatment strategies, and reduces or eliminates the need for "pump and treat", a practice common at sites where hydrocarbons have contaminated clean groundwater.<br /> [edit]Monitoring bioremediation<br /> <br /> The process of bioremediation can be monitored indirectly by measuring the Oxidation Reduction Potential or redox in soil and groundwater, together with pH, temperature, oxygen content, electron acceptor/donor concentrations, and concentration of breakdown products (e.g. carbon dioxide). This table shows the (decreasing) biological breakdown rate as function of the redox potential.<br /> Process Reaction Redox potential (Eh in mV)<br /> aerobic: O2 + 4e− + 4H+ → 2H2O 600 ~ 400<br /> anaerobic: <br /> <br /> denitrification 2NO3− + 10e− + 12H+ → N2 + 6H2O 500 ~ 200<br /> manganese IV reduction MnO2 + 2e− + 4H+ → Mn2+ + 2H2O 400 ~ 200<br /> iron III reduction Fe(OH)3 + e− + 3H+ → Fe2+ + 3H2O 300 ~ 100<br /> sulfate reduction SO42− + 8e− +10 H+ → H2S + 4H2O 0 ~ −150<br /> fermentation 2CH2O → CO2 + CH4 −150 ~ −220<br /> This, by itself and at a single site, gives little information about the process of remediation.<br /> It is necessary to sample enough points on and around the contaminated site to be able to determine contours of equal redox potential. Contouring is usually done using specialised software, e.g. using Kriging interpolation.<br /> If all the measurements of redox potential show that electron acceptors have been used up, it is in effect an indicator for total microbial activity. Chemical analysis is also required to determine when the levels of contaminants and their breakdown products have been reduced to below regulatory limits.<br /> [edit]See also
MH-Miranda House
polymerase chain reaction
The polymerase chain reaction (PCR) is a scientific technique in molecular biology to amplify a single or a few copies of a piece of DNA across several orders of magnitude, generating thousands to millions of copies of a particular DNA sequence.<br /> Developed in 1983 by Kary Mullis,[1] PCR is now a common and often indispensable technique used in medical and biological research labs for a variety of applications.[2][3] These include DNA cloning for sequencing, DNA-based phylogeny, or functional analysis of genes; the diagnosis of hereditary diseases; the identification of genetic fingerprints (used in forensic sciences and paternity testing); and the detection and diagnosis of infectious diseases. In 1993, Mullis was awarded the Nobel Prize in Chemistry along with Michael Smith for his work on PCR.[4]<br /> The method relies on thermal cycling, consisting of cycles of repeated heating and cooling of the reaction for DNA melting and enzymatic replication of the DNA. Primers (short DNA fragments) containing sequences complementary to the target region along with a DNA polymerase (after which the method is named) are key components to enable selective and repeated amplification. As PCR progresses, the DNA generated is itself used as a template for replication, setting in motion a chain reaction in which the DNA template is exponentially amplified. PCR can be extensively modified to perform a wide array of genetic manipulations.<br /> Almost all PCR applications employ a heat-stable DNA polymerase, such as Taq polymerase, an enzyme originally isolated from the bacterium Thermus aquaticus. This DNA polymerase enzymatically assembles a new DNA strand from DNA building-blocks, the nucleotides, by using single-stranded DNA as a template and DNA oligonucleotides (also called DNA primers), which are required for initiation of DNA synthesis. The vast majority of PCR methods use thermal cycling, i.e., alternately heating and cooling the PCR sample to a defined series of temperature steps. These thermal cycling steps are necessary first to physically separate the two strands in a DNA double helix at a high temperature in a process called DNA melting. At a lower temperature, each strand is then used as the template in DNA synthesis by the DNA polymerase to selectively amplify the target DNA. The selectivity of PCR results from the use of primers that are complementary to the DNA region targeted for amplification under specific thermal cycling conditions.<br /> <br /> <br /> Placing a strip of eight PCR tubes, each containing a 100 μl reaction mixture, into the PCR machine<br /> Contents [hide]<br /> 1 PCR principles and procedure<br /> 1.1 Procedure<br /> 2 PCR stages<br /> 2.1 PCR optimization<br /> 3 Application of PCR<br /> 3.1 Selective DNA isolation<br /> 3.2 Amplification and quantification of DNA<br /> 3.3 PCR in diagnosis of diseases<br /> 4 Variations on the basic PCR technique<br /> 5 History<br /> 5.1 Patent wars<br /> 6 References<br /> 7 External links<br /> [edit]PCR principles and procedure<br /> <br /> <br /> <br /> Figure 1a: A thermal cycler for PCR<br /> <br /> <br /> Figure 1b: An older model three-temperature thermal cycler for PCR<br /> PCR is used to amplify a specific region of a DNA strand (the DNA target). Most PCR methods typically amplify DNA fragments of up to ~10 kilo base pairs (kb), although some techniques allow for amplification of fragments up to 40 kb in size.[5]<br /> A basic PCR set up requires several components and reagents.[6] These components include:<br /> DNA template that contains the DNA region (target) to be amplified.<br /> Two primers that are complementary to the 3' (three prime) ends of each of the sense and anti-sense strand of the DNA target.<br /> Taq polymerase or another DNA polymerase with a temperature optimum at around 70 °C.<br /> Deoxynucleoside triphosphates (dNTPs; nucleotides containing triphosphate groups), the building-blocks from which the DNA polymerase synthesizes a new DNA strand.<br /> Buffer solution, providing a suitable chemical environment for optimum activity and stability of the DNA polymerase.<br /> Divalent cations, magnesium or manganese ions; generally Mg2+ is used, but Mn2+ can be utilized for PCR-mediated DNA mutagenesis, as higher Mn2+ concentration increases the error rate during DNA synthesis[7]<br /> Monovalent cation potassium ions.<br /> The PCR is commonly carried out in a reaction volume of 10–200 μl in small reaction tubes (0.2–0.5 ml volumes) in a thermal cycler. The thermal cycler heats and cools the reaction tubes to achieve the temperatures required at each step of the reaction (see below). Many modern thermal cyclers make use of the Peltier effect, which permits both heating and cooling of the block holding the PCR tubes simply by reversing the electric current. Thin-walled reaction tubes permit favorable thermal conductivity to allow for rapid thermal equilibration. Most thermal cyclers have heated lids to prevent condensation at the top of the reaction tube. Older thermocyclers lacking a heated lid require a layer of oil on top of the reaction mixture or a ball of wax inside the tube.<br /> [edit]Procedure<br /> <br /> <br /> Figure 2: Schematic drawing of the PCR cycle. (1) Denaturing at 94–96 °C. (2) Annealing at ~65 °C (3) Elongation at 72 °C. Four cycles are shown here. The blue lines represent the DNA template to which primers (red arrows) anneal that are extended by the DNA polymerase (light green circles), to give shorter DNA products (green lines), which themselves are used as templates as PCR progresses.<br /> Typically, PCR consists of a series of 20-40 repeated temperature changes, called cycles, with each cycle commonly consisting of 2-3 discrete temperature steps, usually three (Fig. 2). The cycling is often preceded by a single temperature step (called hold) at a high temperature (>90°C), and followed by one hold at the end for final product extension or brief storage. The temperatures used and the length of time they are applied in each cycle depend on a variety of parameters. These include the enzyme used for DNA synthesis, the concentration of divalent ions and dNTPs in the reaction, and the melting temperature (Tm) of the primers.[8]<br /> Initialization step: This step consists of heating the reaction to a temperature of 94–96 °C (or 98 °C if extremely thermostable polymerases are used), which is held for 1–9 minutes. It is only required for DNA polymerases that require heat activation by hot-start PCR.[9]<br /> Denaturation step: This step is the first regular cycling event and consists of heating the reaction to 94–98 °C for 20–30 seconds. It causes DNA melting of the DNA template by disrupting the hydrogen bonds between complementary bases, yielding single-stranded DNA molecules.<br /> Annealing step: The reaction temperature is lowered to 50–65 °C for 20–40 seconds allowing annealing of the primers to the single-stranded DNA template. Typically the annealing temperature is about 3-5 degrees Celsius below the Tm of the primers used. Stable DNA-DNA hydrogen bonds are only formed when the primer sequence very closely matches the template sequence. The polymerase binds to the primer-template hybrid and begins DNA formation .<br /> Extension/elongation step: The temperature at this step depends on the DNA polymerase used; Taq polymerase has its optimum activity temperature at 75–80 °C,[10][11] and commonly a temperature of 72 °C is used with this enzyme. At this step the DNA polymerase synthesizes a new DNA strand complementary to the DNA template strand by adding dNTPs that are complementary to the template in 5' to 3' direction, condensing the 5'-phosphate group of the dNTPs with the 3'-hydroxyl group at the end of the nascent (extending) DNA strand. The extension time depends both on the DNA polymerase used and on the length of the DNA fragment to be amplified. As a rule-of-thumb, at its optimum temperature, the DNA polymerase will polymerize a thousand bases per minute. Under optimum conditions, i.e., if there are no limitations due to limiting substrates or reagents, at each extension step, the amount of DNA target is doubled, leading to exponential (geometric) amplification of the specific DNA fragment.<br /> Final elongation: This single step is occasionally performed at a temperature of 70–74 °C for 5–15 minutes after the last PCR cycle to ensure that any remaining single-stranded DNA is fully extended.<br /> Final hold: This step at 4–15 °C for an indefinite time may be employed for short-term storage of the reaction.<br /> <br /> <br /> Figure 3: Ethidium bromide-stained PCR products after gel electrophoresis. Two sets of primers were used to amplify a target sequence from three different tissue samples. No amplification is present in sample #1; DNA bands in sample #2 and #3 indicate successful amplification of the target sequence. The gel also shows a positive control, and a DNA ladder containing DNA fragments of defined length for sizing the bands in the experimental PCRs.<br /> To check whether the PCR generated the anticipated DNA fragment (also sometimes referred to as the amplimer or amplicon), agarose gel electrophoresis is employed for size separation of the PCR products. The size(s) of PCR products is determined by comparison with a DNA ladder (a molecular weight marker), which contains DNA fragments of known size, run on the gel alongside the PCR products (see Fig. 3).<br /> [edit]PCR stages<br /> <br /> The PCR process can be divided into three stages:<br /> Exponential amplification: At every cycle, the amount of product is doubled (assuming 100% reaction efficiency). The reaction is very sensitive: only minute quantities of DNA need to be present.[12]<br /> Leveling off stage: The reaction slows as the DNA polymerase loses activity and as consumption of reagents such as dNTPs and primers causes them to become limiting.<br /> Plateau: No more product accumulates due to exhaustion of reagents and enzyme.<br /> [edit]PCR optimization<br /> Main article: PCR optimization<br /> In practice, PCR can fail for various reasons, in part due to its sensitivity to contamination causing amplification of spurious DNA products. Because of this, a number of techniques and procedures have been developed for optimizing PCR conditions.[13][14] Contamination with extraneous DNA is addressed with lab protocols and procedures that separate pre-PCR mixtures from potential DNA contaminants.[6] This usually involves spatial separation of PCR-setup areas from areas for analysis or purification of PCR products, use of disposable plasticware, and thoroughly cleaning the work surface between reaction setups. Primer-design techniques are important in improving PCR product yield and in avoiding the formation of spurious products, and the usage of alternate buffer components or polymerase enzymes can help with amplification of long or otherwise problematic regions of DNA. Addition of reagents, such as formamide, in buffer systems may increase the specificity and yield of PCR.[15] Computer simulations of theoretical PCR results (Electronic PCR) may be performed to assist in primer design.[16]<br /> [edit]Application of PCR<br /> <br /> Main article: Applications of PCR<br /> [edit]Selective DNA isolation<br /> PCR allows isolation of DNA fragments from genomic DNA by selective amplification of a specific region of DNA. This use of PCR augments many methods, such as generating hybridization probes for Southern or northern hybridization and DNA cloning, which require larger amounts of DNA, representing a specific DNA region. PCR supplies these techniques with high amounts of pure DNA, enabling analysis of DNA samples even from very small amounts of starting material.<br /> Other applications of PCR include DNA sequencing to determine unknown PCR-amplified sequences in which one of the amplification primers may be used in Sanger sequencing, isolation of a DNA sequence to expedite recombinant DNA technologies involving the insertion of a DNA sequence into a plasmid or the genetic material of another organism. Bacterial colonies (E. coli) can be rapidly screened by PCR for correct DNA vector constructs.[17] PCR may also be used for genetic fingerprinting; a forensic technique used to identify a person or organism by comparing experimental DNAs through different PCR-based methods.[citation needed]<br /> Some PCR 'fingerprints' methods have high discriminative power and can be used to identify genetic relationships between individuals, such as parent-child or between siblings, and are used in paternity testing (Fig. 4). This technique may also be used to determine evolutionary relationships among organisms.[citation needed]<br /> <br /> <br /> Figure 4: Electrophoresis of PCR-amplified DNA fragments. (1) Father. (2) Child. (3) Mother. The child has inherited some, but not all of the fingerprint of each of its parents, giving it a new, unique fingerprint.<br /> [edit]Amplification and quantification of DNA<br /> Because PCR amplifies the regions of DNA that it targets, PCR can be used to analyze extremely small amounts of sample. This is often critical for forensic analysis, when only a trace amount of DNA is available as evidence. PCR may also be used in the analysis of ancient DNA that is tens of thousands of years old. These PCR-based techniques have been successfully used on animals, such as a forty-thousand-year-old mammoth, and also on human DNA, in applications ranging from the analysis of Egyptian mummies to the identification of a Russian tsar.[18]<br /> Quantitative PCR methods allow the estimation of the amount of a given sequence present in a sample—a technique often applied to quantitatively determine levels of gene expression. Real-time PCR is an established tool for DNA quantification that measures the accumulation of DNA product after each round of PCR amplification.<br /> See also Use of DNA in forensic entomology<br /> [edit]PCR in diagnosis of diseases<br /> PCR permits early diagnosis of malignant diseases such as leukemia and lymphomas, which is currently the highest-developed in cancer research and is already being used routinely. (See the studies cited in the EUTOS For CML study article at http://www.eutos.org/content/molecular_monitoring/information/pcr_testing/, especially notes 10-13.) PCR assays can be performed directly on genomic DNA samples to detect translocation-specific malignant cells at a sensitivity that is at least 10,000-fold higher than that of other methods.[citation needed]<br /> PCR also permits identification of non-cultivatable or slow-growing microorganisms such as mycobacteria, anaerobic bacteria, or viruses from tissue culture assays and animal models. The basis for PCR diagnostic applications in microbiology is the detection of infectious agents and the discrimination of non-pathogenic from pathogenic strains by virtue of specific genes.[19]<br /> Viral DNA can likewise be detected by PCR. The primers used need to be specific to the targeted sequences in the DNA of a virus, and the PCR can be used for diagnostic analyses or DNA sequencing of the viral genome. The high sensitivity of PCR permits virus detection soon after infection and even before the onset of disease. Such early detection may give physicians a significant lead in treatment. The amount of virus ("viral load") in a patient can also be quantified by PCR-based DNA quantitation techniques (see below).<br /> [edit]Variations on the basic PCR technique<br /> <br /> Main article: Variants of PCR<br /> Allele-specific PCR: a diagnostic or cloning technique based on single-nucleotide polymorphisms (SNPs) (single-base differences in DNA). It requires prior knowledge of a DNA sequence, including differences between alleles, and uses primers whose 3' ends encompass the SNP. PCR amplification under stringent conditions is much less efficient in the presence of a mismatch between template and primer, so successful amplification with an SNP-specific primer signals presence of the specific SNP in a sequence.[20] See SNP genotyping for more information.<br /> Assembly PCR or Polymerase Cycling Assembly (PCA): artificial synthesis of long DNA sequences by performing PCR on a pool of long oligonucleotides with short overlapping segments. The oligonucleotides alternate between sense and antisense directions, and the overlapping segments determine the order of the PCR fragments, thereby selectively producing the final long DNA product.[21]<br /> Asymmetric PCR: preferentially amplifies one DNA strand in a double-stranded DNA template. It is used in sequencing and hybridization probing where amplification of only one of the two complementary strands is required. PCR is carried out as usual, but with a great excess of the primer for the strand targeted for amplification. Because of the slow (arithmetic) amplification later in the reaction after the limiting primer has been used up, extra cycles of PCR are required.[22] A recent modification on this process, known as Linear-After-The-Exponential-PCR (LATE-PCR), uses a limiting primer with a higher melting temperature (Tm) than the excess primer to maintain reaction efficiency as the limiting primer concentration decreases mid-reaction.[23]<br /> Helicase-dependent amplification: similar to traditional PCR, but uses a constant temperature rather than cycling through denaturation and annealing/extension cycles. DNA helicase, an enzyme that unwinds DNA, is used in place of thermal denaturation.[24]<br /> Hot start PCR: a technique that reduces non-specific amplification during the initial set up stages of the PCR. It may be performed manually by heating the reaction components to the denaturation temperature (e.g., 95°C) before adding the polymerase.[25] Specialized enzyme systems have been developed that inhibit the polymerase's activity at ambient temperature, either by the binding of an antibody[9][26] or by the presence of covalently bound inhibitors that dissociate only after a high-temperature activation step. Hot-start/cold-finish PCR is achieved with new hybrid polymerases that are inactive at ambient temperature and are instantly activated at elongation temperature.<br /> Intersequence-specific PCR (ISSR): a PCR method for DNA fingerprinting that amplifies regions between simple sequence repeats to produce a unique fingerprint of amplified fragment lengths.[27]<br /> Inverse PCR: is commonly used to identify the flanking sequences around genomic inserts. It involves a series of DNA digestions and self ligation, resulting in known sequences at either end of the unknown sequence.[28]<br /> Ligation-mediated PCR: uses small DNA linkers ligated to the DNA of interest and multiple primers annealing to the DNA linkers; it has been used for DNA sequencing, genome walking, and DNA footprinting.[29]<br /> Methylation-specific PCR (MSP): developed by Stephen Baylin and Jim Herman at the Johns Hopkins School of Medicine,[30] and is used to detect methylation of CpG islands in genomic DNA. DNA is first treated with sodium bisulfite, which converts unmethylated cytosine bases to uracil, which is recognized by PCR primers as thymine. Two PCRs are then carried out on the modified DNA, using primer sets identical except at any CpG islands within the primer sequences. At these points, one primer set recognizes DNA with cytosines to amplify methylated DNA, and one set recognizes DNA with uracil or thymine to amplify unmethylated DNA. MSP using qPCR can also be performed to obtain quantitative rather than qualitative information about methylation.<br /> Miniprimer PCR: uses a thermostable polymerase (S-Tbr) that can extend from short primers ("smalligos") as short as 9 or 10 nucleotides. This method permits PCR targeting to smaller primer binding regions, and is used to amplify conserved DNA sequences, such as the 16S (or eukaryotic 18S) rRNA gene.[31]<br /> Multiplex Ligation-dependent Probe Amplification (MLPA): permits multiple targets to be amplified with only a single primer pair, thus avoiding the resolution limitations of multiplex PCR (see below).<br /> Multiplex-PCR: consists of multiple primer sets within a single PCR mixture to produce amplicons of varying sizes that are specific to different DNA sequences. By targeting multiple genes at once, additional information may be gained from a single test-run that otherwise would require several times the reagents and more time to perform. Annealing temperatures for each of the primer sets must be optimized to work correctly within a single reaction, and amplicon sizes. That is, their base pair length should be different enough to form distinct bands when visualized by gel electrophoresis.<br /> Nested PCR: increases the specificity of DNA amplification, by reducing background due to non-specific amplification of DNA. Two sets of primers are used in two successive PCRs. In the first reaction, one pair of primers is used to generate DNA products, which besides the intended target, may still consist of non-specifically amplified DNA fragments. The product(s) are then used in a second PCR with a set of primers whose binding sites are completely or partially different from and located 3' of each of the primers used in the first reaction. Nested PCR is often more successful in specifically amplifying long DNA fragments than conventional PCR, but it requires more detailed knowledge of the target sequences.<br /> Overlap-extension PCR or Splicing by overlap extension (SOE) : a genetic engineering technique that is used to splice together two or more DNA fragments that contain complementary sequences. It is used to join DNA pieces containing genes, regulatory sequences, or mutations; the technique enables creation of specific and long DNA constructs.<br /> Quantitative PCR (Q-PCR): used to measure the quantity of a PCR product (commonly in real-time). It quantitatively measures starting amounts of DNA, cDNA, or RNA. Q-PCR is commonly used to determine whether a DNA sequence is present in a sample and the number of its copies in the sample. Quantitative real-time PCR has a very high degree of precision. QRT-PCR (or QF-PCR) methods use fluorescent dyes, such as Sybr Green, EvaGreen or fluorophore-containing DNA probes, such as TaqMan, to measure the amount of amplified product in real time. It is also sometimes abbreviated to RT-PCR (Real Time PCR) or RQ-PCR. QRT-PCR or RTQ-PCR are more appropriate contractions, since RT-PCR commonly refers to reverse transcription PCR (see below), often used in conjunction with Q-PCR.<br /> Reverse Transcription PCR (RT-PCR): for amplifying DNA from RNA. Reverse transcriptase reverse transcribes RNA into cDNA, which is then amplified by PCR. RT-PCR is widely used in expression profiling, to determine the expression of a gene or to identify the sequence of an RNA transcript, including transcription start and termination sites. If the genomic DNA sequence of a gene is known, RT-PCR can be used to map the location of exons and introns in the gene. The 5' end of a gene (corresponding to the transcription start site) is typically identified by RACE-PCR (Rapid Amplification of cDNA Ends).<br /> Solid Phase PCR: encompasses multiple meanings, including Polony Amplification (where PCR colonies are derived in a gel matrix, for example), Bridge PCR[32] (primers are covalently linked to a solid-support surface), conventional Solid Phase PCR (where Asymmetric PCR is applied in the presence of solid support bearing primer with sequence matching one of the aqueous primers) and Enhanced Solid Phase PCR[33] (where conventional Solid Phase PCR can be improved by employing high Tm and nested solid support primer with optional application of a thermal 'step' to favour solid support priming).<br /> Thermal asymmetric interlaced PCR (TAIL-PCR): for isolation of an unknown sequence flanking a known sequence. Within the known sequence, TAIL-PCR uses a nested pair of primers with differing annealing temperatures; a degenerate primer is used to amplify in the other direction from the unknown sequence.[34]<br /> Touchdown PCR (Step-down PCR): a variant of PCR that aims to reduce nonspecific background by gradually lowering the annealing temperature as PCR cycling progresses. The annealing temperature at the initial cycles is usually a few degrees (3-5°C) above the Tm of the primers used, while at the later cycles, it is a few degrees (3-5°C) below the primer Tm. The higher temperatures give greater specificity for primer binding, and the lower temperatures permit more efficient amplification from the specific products formed during the initial cycles.[35]<br /> PAN-AC: uses isothermal conditions for amplification, and may be used in living cells.[36][37]<br /> Universal Fast Walking: for genome walking and genetic fingerprinting using a more specific 'two-sided' PCR than conventional 'one-sided' approaches (using only one gene-specific primer and one general primer — which can lead to artefactual 'noise')[38] by virtue of a mechanism involving lariat structure formation. Streamlined derivatives of UFW are LaNe RAGE (lariat-dependent nested PCR for rapid amplification of genomic DNA ends),[39] 5'RACE LaNe[40] and 3'RACE LaNe.[41]<br /> In silico PCR (digital PCR, virtual PCR, electronic PCR, e-PCR) refers to computational tools used to calculate theoretical polymerase chain reaction results using a given set of primers (probes) to amplify DNA sequences from a sequenced genome or transcriptome.<br /> <br /> [edit]History<br /> <br /> Main article: History of polymerase chain reaction<br /> A 1971 paper in the Journal of Molecular Biology by Kleppe and co-workers first described a method using an enzymatic assay to replicate a short DNA template with primers in vitro.[42] However, this early manifestation of the basic PCR principle did not receive much attention, and the invention of the polymerase chain reaction in 1983 is generally credited to Kary Mullis.[43]<br /> When Mullis developed the PCR in 1983, he was working in Emeryville, California for Cetus Corporation, one of the first biotechnology companies. There, he was responsible for synthesizing short chains of DNA. Mullis has written that he conceived of PCR while cruising along the Pacific Coast Highway one night in his car.[44] He was playing in his mind with a new way of analyzing changes (mutations) in DNA when he realized that he had instead invented a method of amplifying any DNA region through repeated cycles of duplication driven by DNA polymerase. In Scientific American, Mullis summarized the procedure: "Beginning with a single molecule of the genetic material DNA, the PCR can generate 100 billion similar molecules in an afternoon. The reaction is easy to execute. It requires no more than a test tube, a few simple reagents, and a source of heat."[45] He was awarded the Nobel Prize in Chemistry in 1993 for his invention,[4] seven years after he and his colleagues at Cetus first put his proposal to practice. However, some controversies have remained about the intellectual and practical contributions of other scientists to Mullis' work, and whether he had been the sole inventor of the PCR principle (see below).<br /> At the core of the PCR method is the use of a suitable DNA polymerase able to withstand the high temperatures of >90 °C (194 °F) required for separation of the two DNA strands in the DNA double helix after each replication cycle. The DNA polymerases initially employed for in vitro experiments presaging PCR were unable to withstand these high temperatures.[2] So the early procedures for DNA replication were very inefficient and time consuming, and required large amounts of DNA polymerase and continuous handling throughout the process.<br /> The discovery in 1976 of Taq polymerase — a DNA polymerase purified from the thermophilic bacterium, Thermus aquaticus, which naturally lives in hot (50 to 80 °C (122 to 176 °F)) environments[10] such as hot springs — paved the way for dramatic improvements of the PCR method. The DNA polymerase isolated from T. aquaticus is stable at high temperatures remaining active even after DNA denaturation,[11] thus obviating the need to add new DNA polymerase after each cycle.[3] This allowed an automated thermocycler-based process for DNA amplification.<br /> [edit]Patent wars<br /> The PCR technique was patented by Kary Mullis and assigned to Cetus Corporation, where Mullis worked when he invented the technique in 1983. The Taq polymerase enzyme was also covered by patents. There have been several high-profile lawsuits related to the technique, including an unsuccessful lawsuit brought by DuPont. The pharmaceutical company Hoffmann-La Roche purchased the rights to the patents in 1992 and currently holds those that are still protected.<br /> A related patent battle over the Taq polymerase enzyme is still ongoing in several jurisdictions around the world between Roche and Promega. The legal arguments have extended beyond the lives of the original PCR and Taq polymerase patents, which expired on March 28, 2005.
MH-Miranda House
polymerase chain reaction
The polymerase chain reaction (PCR) is a scientific technique in molecular biology to amplify a single or a few copies of a piece of DNA across several orders of magnitude, generating thousands to millions of copies of a particular DNA sequence.<br /> Developed in 1983 by Kary Mullis,[1] PCR is now a common and often indispensable technique used in medical and biological research labs for a variety of applications.[2][3] These include DNA cloning for sequencing, DNA-based phylogeny, or functional analysis of genes; the diagnosis of hereditary diseases; the identification of genetic fingerprints (used in forensic sciences and paternity testing); and the detection and diagnosis of infectious diseases. In 1993, Mullis was awarded the Nobel Prize in Chemistry along with Michael Smith for his work on PCR.[4]<br /> The method relies on thermal cycling, consisting of cycles of repeated heating and cooling of the reaction for DNA melting and enzymatic replication of the DNA. Primers (short DNA fragments) containing sequences complementary to the target region along with a DNA polymerase (after which the method is named) are key components to enable selective and repeated amplification. As PCR progresses, the DNA generated is itself used as a template for replication, setting in motion a chain reaction in which the DNA template is exponentially amplified. PCR can be extensively modified to perform a wide array of genetic manipulations.<br /> Almost all PCR applications employ a heat-stable DNA polymerase, such as Taq polymerase, an enzyme originally isolated from the bacterium Thermus aquaticus. This DNA polymerase enzymatically assembles a new DNA strand from DNA building-blocks, the nucleotides, by using single-stranded DNA as a template and DNA oligonucleotides (also called DNA primers), which are required for initiation of DNA synthesis. The vast majority of PCR methods use thermal cycling, i.e., alternately heating and cooling the PCR sample to a defined series of temperature steps. These thermal cycling steps are necessary first to physically separate the two strands in a DNA double helix at a high temperature in a process called DNA melting. At a lower temperature, each strand is then used as the template in DNA synthesis by the DNA polymerase to selectively amplify the target DNA. The selectivity of PCR results from the use of primers that are complementary to the DNA region targeted for amplification under specific thermal cycling conditions.<br /> <br /> <br /> Placing a strip of eight PCR tubes, each containing a 100 μl reaction mixture, into the PCR machine<br /> Contents [hide]<br /> 1 PCR principles and procedure<br /> 1.1 Procedure<br /> 2 PCR stages<br /> 2.1 PCR optimization<br /> 3 Application of PCR<br /> 3.1 Selective DNA isolation<br /> 3.2 Amplification and quantification of DNA<br /> 3.3 PCR in diagnosis of diseases<br /> 4 Variations on the basic PCR technique<br /> 5 History<br /> 5.1 Patent wars<br /> 6 References<br /> 7 External links<br /> [edit]PCR principles and procedure<br /> <br /> <br /> <br /> Figure 1a: A thermal cycler for PCR<br /> <br /> <br /> Figure 1b: An older model three-temperature thermal cycler for PCR<br /> PCR is used to amplify a specific region of a DNA strand (the DNA target). Most PCR methods typically amplify DNA fragments of up to ~10 kilo base pairs (kb), although some techniques allow for amplification of fragments up to 40 kb in size.[5]<br /> A basic PCR set up requires several components and reagents.[6] These components include:<br /> DNA template that contains the DNA region (target) to be amplified.<br /> Two primers that are complementary to the 3' (three prime) ends of each of the sense and anti-sense strand of the DNA target.<br /> Taq polymerase or another DNA polymerase with a temperature optimum at around 70 °C.<br /> Deoxynucleoside triphosphates (dNTPs; nucleotides containing triphosphate groups), the building-blocks from which the DNA polymerase synthesizes a new DNA strand.<br /> Buffer solution, providing a suitable chemical environment for optimum activity and stability of the DNA polymerase.<br /> Divalent cations, magnesium or manganese ions; generally Mg2+ is used, but Mn2+ can be utilized for PCR-mediated DNA mutagenesis, as higher Mn2+ concentration increases the error rate during DNA synthesis[7]<br /> Monovalent cation potassium ions.<br /> The PCR is commonly carried out in a reaction volume of 10–200 μl in small reaction tubes (0.2–0.5 ml volumes) in a thermal cycler. The thermal cycler heats and cools the reaction tubes to achieve the temperatures required at each step of the reaction (see below). Many modern thermal cyclers make use of the Peltier effect, which permits both heating and cooling of the block holding the PCR tubes simply by reversing the electric current. Thin-walled reaction tubes permit favorable thermal conductivity to allow for rapid thermal equilibration. Most thermal cyclers have heated lids to prevent condensation at the top of the reaction tube. Older thermocyclers lacking a heated lid require a layer of oil on top of the reaction mixture or a ball of wax inside the tube.<br /> [edit]Procedure<br /> <br /> <br /> Figure 2: Schematic drawing of the PCR cycle. (1) Denaturing at 94–96 °C. (2) Annealing at ~65 °C (3) Elongation at 72 °C. Four cycles are shown here. The blue lines represent the DNA template to which primers (red arrows) anneal that are extended by the DNA polymerase (light green circles), to give shorter DNA products (green lines), which themselves are used as templates as PCR progresses.<br /> Typically, PCR consists of a series of 20-40 repeated temperature changes, called cycles, with each cycle commonly consisting of 2-3 discrete temperature steps, usually three (Fig. 2). The cycling is often preceded by a single temperature step (called hold) at a high temperature (>90°C), and followed by one hold at the end for final product extension or brief storage. The temperatures used and the length of time they are applied in each cycle depend on a variety of parameters. These include the enzyme used for DNA synthesis, the concentration of divalent ions and dNTPs in the reaction, and the melting temperature (Tm) of the primers.[8]<br /> Initialization step: This step consists of heating the reaction to a temperature of 94–96 °C (or 98 °C if extremely thermostable polymerases are used), which is held for 1–9 minutes. It is only required for DNA polymerases that require heat activation by hot-start PCR.[9]<br /> Denaturation step: This step is the first regular cycling event and consists of heating the reaction to 94–98 °C for 20–30 seconds. It causes DNA melting of the DNA template by disrupting the hydrogen bonds between complementary bases, yielding single-stranded DNA molecules.<br /> Annealing step: The reaction temperature is lowered to 50–65 °C for 20–40 seconds allowing annealing of the primers to the single-stranded DNA template. Typically the annealing temperature is about 3-5 degrees Celsius below the Tm of the primers used. Stable DNA-DNA hydrogen bonds are only formed when the primer sequence very closely matches the template sequence. The polymerase binds to the primer-template hybrid and begins DNA formation .<br /> Extension/elongation step: The temperature at this step depends on the DNA polymerase used; Taq polymerase has its optimum activity temperature at 75–80 °C,[10][11] and commonly a temperature of 72 °C is used with this enzyme. At this step the DNA polymerase synthesizes a new DNA strand complementary to the DNA template strand by adding dNTPs that are complementary to the template in 5' to 3' direction, condensing the 5'-phosphate group of the dNTPs with the 3'-hydroxyl group at the end of the nascent (extending) DNA strand. The extension time depends both on the DNA polymerase used and on the length of the DNA fragment to be amplified. As a rule-of-thumb, at its optimum temperature, the DNA polymerase will polymerize a thousand bases per minute. Under optimum conditions, i.e., if there are no limitations due to limiting substrates or reagents, at each extension step, the amount of DNA target is doubled, leading to exponential (geometric) amplification of the specific DNA fragment.<br /> Final elongation: This single step is occasionally performed at a temperature of 70–74 °C for 5–15 minutes after the last PCR cycle to ensure that any remaining single-stranded DNA is fully extended.<br /> Final hold: This step at 4–15 °C for an indefinite time may be employed for short-term storage of the reaction.<br /> <br /> <br /> Figure 3: Ethidium bromide-stained PCR products after gel electrophoresis. Two sets of primers were used to amplify a target sequence from three different tissue samples. No amplification is present in sample #1; DNA bands in sample #2 and #3 indicate successful amplification of the target sequence. The gel also shows a positive control, and a DNA ladder containing DNA fragments of defined length for sizing the bands in the experimental PCRs.<br /> To check whether the PCR generated the anticipated DNA fragment (also sometimes referred to as the amplimer or amplicon), agarose gel electrophoresis is employed for size separation of the PCR products. The size(s) of PCR products is determined by comparison with a DNA ladder (a molecular weight marker), which contains DNA fragments of known size, run on the gel alongside the PCR products (see Fig. 3).<br /> [edit]PCR stages<br /> <br /> The PCR process can be divided into three stages:<br /> Exponential amplification: At every cycle, the amount of product is doubled (assuming 100% reaction efficiency). The reaction is very sensitive: only minute quantities of DNA need to be present.[12]<br /> Leveling off stage: The reaction slows as the DNA polymerase loses activity and as consumption of reagents such as dNTPs and primers causes them to become limiting.<br /> Plateau: No more product accumulates due to exhaustion of reagents and enzyme.<br /> [edit]PCR optimization<br /> Main article: PCR optimization<br /> In practice, PCR can fail for various reasons, in part due to its sensitivity to contamination causing amplification of spurious DNA products. Because of this, a number of techniques and procedures have been developed for optimizing PCR conditions.[13][14] Contamination with extraneous DNA is addressed with lab protocols and procedures that separate pre-PCR mixtures from potential DNA contaminants.[6] This usually involves spatial separation of PCR-setup areas from areas for analysis or purification of PCR products, use of disposable plasticware, and thoroughly cleaning the work surface between reaction setups. Primer-design techniques are important in improving PCR product yield and in avoiding the formation of spurious products, and the usage of alternate buffer components or polymerase enzymes can help with amplification of long or otherwise problematic regions of DNA. Addition of reagents, such as formamide, in buffer systems may increase the specificity and yield of PCR.[15] Computer simulations of theoretical PCR results (Electronic PCR) may be performed to assist in primer design.[16]<br /> [edit]Application of PCR<br /> <br /> Main article: Applications of PCR<br /> [edit]Selective DNA isolation<br /> PCR allows isolation of DNA fragments from genomic DNA by selective amplification of a specific region of DNA. This use of PCR augments many methods, such as generating hybridization probes for Southern or northern hybridization and DNA cloning, which require larger amounts of DNA, representing a specific DNA region. PCR supplies these techniques with high amounts of pure DNA, enabling analysis of DNA samples even from very small amounts of starting material.<br /> Other applications of PCR include DNA sequencing to determine unknown PCR-amplified sequences in which one of the amplification primers may be used in Sanger sequencing, isolation of a DNA sequence to expedite recombinant DNA technologies involving the insertion of a DNA sequence into a plasmid or the genetic material of another organism. Bacterial colonies (E. coli) can be rapidly screened by PCR for correct DNA vector constructs.[17] PCR may also be used for genetic fingerprinting; a forensic technique used to identify a person or organism by comparing experimental DNAs through different PCR-based methods.[citation needed]<br /> Some PCR 'fingerprints' methods have high discriminative power and can be used to identify genetic relationships between individuals, such as parent-child or between siblings, and are used in paternity testing (Fig. 4). This technique may also be used to determine evolutionary relationships among organisms.[citation needed]<br /> <br /> <br /> Figure 4: Electrophoresis of PCR-amplified DNA fragments. (1) Father. (2) Child. (3) Mother. The child has inherited some, but not all of the fingerprint of each of its parents, giving it a new, unique fingerprint.<br /> [edit]Amplification and quantification of DNA<br /> Because PCR amplifies the regions of DNA that it targets, PCR can be used to analyze extremely small amounts of sample. This is often critical for forensic analysis, when only a trace amount of DNA is available as evidence. PCR may also be used in the analysis of ancient DNA that is tens of thousands of years old. These PCR-based techniques have been successfully used on animals, such as a forty-thousand-year-old mammoth, and also on human DNA, in applications ranging from the analysis of Egyptian mummies to the identification of a Russian tsar.[18]<br /> Quantitative PCR methods allow the estimation of the amount of a given sequence present in a sample—a technique often applied to quantitatively determine levels of gene expression. Real-time PCR is an established tool for DNA quantification that measures the accumulation of DNA product after each round of PCR amplification.<br /> See also Use of DNA in forensic entomology<br /> [edit]PCR in diagnosis of diseases<br /> PCR permits early diagnosis of malignant diseases such as leukemia and lymphomas, which is currently the highest-developed in cancer research and is already being used routinely. (See the studies cited in the EUTOS For CML study article at http://www.eutos.org/content/molecular_monitoring/information/pcr_testing/, especially notes 10-13.) PCR assays can be performed directly on genomic DNA samples to detect translocation-specific malignant cells at a sensitivity that is at least 10,000-fold higher than that of other methods.[citation needed]<br /> PCR also permits identification of non-cultivatable or slow-growing microorganisms such as mycobacteria, anaerobic bacteria, or viruses from tissue culture assays and animal models. The basis for PCR diagnostic applications in microbiology is the detection of infectious agents and the discrimination of non-pathogenic from pathogenic strains by virtue of specific genes.[19]<br /> Viral DNA can likewise be detected by PCR. The primers used need to be specific to the targeted sequences in the DNA of a virus, and the PCR can be used for diagnostic analyses or DNA sequencing of the viral genome. The high sensitivity of PCR permits virus detection soon after infection and even before the onset of disease. Such early detection may give physicians a significant lead in treatment. The amount of virus ("viral load") in a patient can also be quantified by PCR-based DNA quantitation techniques (see below).<br /> [edit]Variations on the basic PCR technique<br /> <br /> Main article: Variants of PCR<br /> Allele-specific PCR: a diagnostic or cloning technique based on single-nucleotide polymorphisms (SNPs) (single-base differences in DNA). It requires prior knowledge of a DNA sequence, including differences between alleles, and uses primers whose 3' ends encompass the SNP. PCR amplification under stringent conditions is much less efficient in the presence of a mismatch between template and primer, so successful amplification with an SNP-specific primer signals presence of the specific SNP in a sequence.[20] See SNP genotyping for more information.<br /> Assembly PCR or Polymerase Cycling Assembly (PCA): artificial synthesis of long DNA sequences by performing PCR on a pool of long oligonucleotides with short overlapping segments. The oligonucleotides alternate between sense and antisense directions, and the overlapping segments determine the order of the PCR fragments, thereby selectively producing the final long DNA product.[21]<br /> Asymmetric PCR: preferentially amplifies one DNA strand in a double-stranded DNA template. It is used in sequencing and hybridization probing where amplification of only one of the two complementary strands is required. PCR is carried out as usual, but with a great excess of the primer for the strand targeted for amplification. Because of the slow (arithmetic) amplification later in the reaction after the limiting primer has been used up, extra cycles of PCR are required.[22] A recent modification on this process, known as Linear-After-The-Exponential-PCR (LATE-PCR), uses a limiting primer with a higher melting temperature (Tm) than the excess primer to maintain reaction efficiency as the limiting primer concentration decreases mid-reaction.[23]<br /> Helicase-dependent amplification: similar to traditional PCR, but uses a constant temperature rather than cycling through denaturation and annealing/extension cycles. DNA helicase, an enzyme that unwinds DNA, is used in place of thermal denaturation.[24]<br /> Hot start PCR: a technique that reduces non-specific amplification during the initial set up stages of the PCR. It may be performed manually by heating the reaction components to the denaturation temperature (e.g., 95°C) before adding the polymerase.[25] Specialized enzyme systems have been developed that inhibit the polymerase's activity at ambient temperature, either by the binding of an antibody[9][26] or by the presence of covalently bound inhibitors that dissociate only after a high-temperature activation step. Hot-start/cold-finish PCR is achieved with new hybrid polymerases that are inactive at ambient temperature and are instantly activated at elongation temperature.<br /> Intersequence-specific PCR (ISSR): a PCR method for DNA fingerprinting that amplifies regions between simple sequence repeats to produce a unique fingerprint of amplified fragment lengths.[27]<br /> Inverse PCR: is commonly used to identify the flanking sequences around genomic inserts. It involves a series of DNA digestions and self ligation, resulting in known sequences at either end of the unknown sequence.[28]<br /> Ligation-mediated PCR: uses small DNA linkers ligated to the DNA of interest and multiple primers annealing to the DNA linkers; it has been used for DNA sequencing, genome walking, and DNA footprinting.[29]<br /> Methylation-specific PCR (MSP): developed by Stephen Baylin and Jim Herman at the Johns Hopkins School of Medicine,[30] and is used to detect methylation of CpG islands in genomic DNA. DNA is first treated with sodium bisulfite, which converts unmethylated cytosine bases to uracil, which is recognized by PCR primers as thymine. Two PCRs are then carried out on the modified DNA, using primer sets identical except at any CpG islands within the primer sequences. At these points, one primer set recognizes DNA with cytosines to amplify methylated DNA, and one set recognizes DNA with uracil or thymine to amplify unmethylated DNA. MSP using qPCR can also be performed to obtain quantitative rather than qualitative information about methylation.<br /> Miniprimer PCR: uses a thermostable polymerase (S-Tbr) that can extend from short primers ("smalligos") as short as 9 or 10 nucleotides. This method permits PCR targeting to smaller primer binding regions, and is used to amplify conserved DNA sequences, such as the 16S (or eukaryotic 18S) rRNA gene.[31]<br /> Multiplex Ligation-dependent Probe Amplification (MLPA): permits multiple targets to be amplified with only a single primer pair, thus avoiding the resolution limitations of multiplex PCR (see below).<br /> Multiplex-PCR: consists of multiple primer sets within a single PCR mixture to produce amplicons of varying sizes that are specific to different DNA sequences. By targeting multiple genes at once, additional information may be gained from a single test-run that otherwise would require several times the reagents and more time to perform. Annealing temperatures for each of the primer sets must be optimized to work correctly within a single reaction, and amplicon sizes. That is, their base pair length should be different enough to form distinct bands when visualized by gel electrophoresis.<br /> Nested PCR: increases the specificity of DNA amplification, by reducing background due to non-specific amplification of DNA. Two sets of primers are used in two successive PCRs. In the first reaction, one pair of primers is used to generate DNA products, which besides the intended target, may still consist of non-specifically amplified DNA fragments. The product(s) are then used in a second PCR with a set of primers whose binding sites are completely or partially different from and located 3' of each of the primers used in the first reaction. Nested PCR is often more successful in specifically amplifying long DNA fragments than conventional PCR, but it requires more detailed knowledge of the target sequences.<br /> Overlap-extension PCR or Splicing by overlap extension (SOE) : a genetic engineering technique that is used to splice together two or more DNA fragments that contain complementary sequences. It is used to join DNA pieces containing genes, regulatory sequences, or mutations; the technique enables creation of specific and long DNA constructs.<br /> Quantitative PCR (Q-PCR): used to measure the quantity of a PCR product (commonly in real-time). It quantitatively measures starting amounts of DNA, cDNA, or RNA. Q-PCR is commonly used to determine whether a DNA sequence is present in a sample and the number of its copies in the sample. Quantitative real-time PCR has a very high degree of precision. QRT-PCR (or QF-PCR) methods use fluorescent dyes, such as Sybr Green, EvaGreen or fluorophore-containing DNA probes, such as TaqMan, to measure the amount of amplified product in real time. It is also sometimes abbreviated to RT-PCR (Real Time PCR) or RQ-PCR. QRT-PCR or RTQ-PCR are more appropriate contractions, since RT-PCR commonly refers to reverse transcription PCR (see below), often used in conjunction with Q-PCR.<br /> Reverse Transcription PCR (RT-PCR): for amplifying DNA from RNA. Reverse transcriptase reverse transcribes RNA into cDNA, which is then amplified by PCR. RT-PCR is widely used in expression profiling, to determine the expression of a gene or to identify the sequence of an RNA transcript, including transcription start and termination sites. If the genomic DNA sequence of a gene is known, RT-PCR can be used to map the location of exons and introns in the gene. The 5' end of a gene (corresponding to the transcription start site) is typically identified by RACE-PCR (Rapid Amplification of cDNA Ends).<br /> Solid Phase PCR: encompasses multiple meanings, including Polony Amplification (where PCR colonies are derived in a gel matrix, for example), Bridge PCR[32] (primers are covalently linked to a solid-support surface), conventional Solid Phase PCR (where Asymmetric PCR is applied in the presence of solid support bearing primer with sequence matching one of the aqueous primers) and Enhanced Solid Phase PCR[33] (where conventional Solid Phase PCR can be improved by employing high Tm and nested solid support primer with optional application of a thermal 'step' to favour solid support priming).<br /> Thermal asymmetric interlaced PCR (TAIL-PCR): for isolation of an unknown sequence flanking a known sequence. Within the known sequence, TAIL-PCR uses a nested pair of primers with differing annealing temperatures; a degenerate primer is used to amplify in the other direction from the unknown sequence.[34]<br /> Touchdown PCR (Step-down PCR): a variant of PCR that aims to reduce nonspecific background by gradually lowering the annealing temperature as PCR cycling progresses. The annealing temperature at the initial cycles is usually a few degrees (3-5°C) above the Tm of the primers used, while at the later cycles, it is a few degrees (3-5°C) below the primer Tm. The higher temperatures give greater specificity for primer binding, and the lower temperatures permit more efficient amplification from the specific products formed during the initial cycles.[35]<br /> PAN-AC: uses isothermal conditions for amplification, and may be used in living cells.[36][37]<br /> Universal Fast Walking: for genome walking and genetic fingerprinting using a more specific 'two-sided' PCR than conventional 'one-sided' approaches (using only one gene-specific primer and one general primer — which can lead to artefactual 'noise')[38] by virtue of a mechanism involving lariat structure formation. Streamlined derivatives of UFW are LaNe RAGE (lariat-dependent nested PCR for rapid amplification of genomic DNA ends),[39] 5'RACE LaNe[40] and 3'RACE LaNe.[41]<br /> In silico PCR (digital PCR, virtual PCR, electronic PCR, e-PCR) refers to computational tools used to calculate theoretical polymerase chain reaction results using a given set of primers (probes) to amplify DNA sequences from a sequenced genome or transcriptome.<br /> <br /> [edit]History<br /> <br /> Main article: History of polymerase chain reaction<br /> A 1971 paper in the Journal of Molecular Biology by Kleppe and co-workers first described a method using an enzymatic assay to replicate a short DNA template with primers in vitro.[42] However, this early manifestation of the basic PCR principle did not receive much attention, and the invention of the polymerase chain reaction in 1983 is generally credited to Kary Mullis.[43]<br /> When Mullis developed the PCR in 1983, he was working in Emeryville, California for Cetus Corporation, one of the first biotechnology companies. There, he was responsible for synthesizing short chains of DNA. Mullis has written that he conceived of PCR while cruising along the Pacific Coast Highway one night in his car.[44] He was playing in his mind with a new way of analyzing changes (mutations) in DNA when he realized that he had instead invented a method of amplifying any DNA region through repeated cycles of duplication driven by DNA polymerase. In Scientific American, Mullis summarized the procedure: "Beginning with a single molecule of the genetic material DNA, the PCR can generate 100 billion similar molecules in an afternoon. The reaction is easy to execute. It requires no more than a test tube, a few simple reagents, and a source of heat."[45] He was awarded the Nobel Prize in Chemistry in 1993 for his invention,[4] seven years after he and his colleagues at Cetus first put his proposal to practice. However, some controversies have remained about the intellectual and practical contributions of other scientists to Mullis' work, and whether he had been the sole inventor of the PCR principle (see below).<br /> At the core of the PCR method is the use of a suitable DNA polymerase able to withstand the high temperatures of >90 °C (194 °F) required for separation of the two DNA strands in the DNA double helix after each replication cycle. The DNA polymerases initially employed for in vitro experiments presaging PCR were unable to withstand these high temperatures.[2] So the early procedures for DNA replication were very inefficient and time consuming, and required large amounts of DNA polymerase and continuous handling throughout the process.<br /> The discovery in 1976 of Taq polymerase — a DNA polymerase purified from the thermophilic bacterium, Thermus aquaticus, which naturally lives in hot (50 to 80 °C (122 to 176 °F)) environments[10] such as hot springs — paved the way for dramatic improvements of the PCR method. The DNA polymerase isolated from T. aquaticus is stable at high temperatures remaining active even after DNA denaturation,[11] thus obviating the need to add new DNA polymerase after each cycle.[3] This allowed an automated thermocycler-based process for DNA amplification.<br /> [edit]Patent wars<br /> The PCR technique was patented by Kary Mullis and assigned to Cetus Corporation, where Mullis worked when he invented the technique in 1983. The Taq polymerase enzyme was also covered by patents. There have been several high-profile lawsuits related to the technique, including an unsuccessful lawsuit brought by DuPont. The pharmaceutical company Hoffmann-La Roche purchased the rights to the patents in 1992 and currently holds those that are still protected.<br /> A related patent battle over the Taq polymerase enzyme is still ongoing in several jurisdictions around the world between Roche and Promega. The legal arguments have extended beyond the lives of the original PCR and Taq polymerase patents, which expired on March 28, 2005.
SC-Surana College
FOREX
PPT
Other
international business
notes pdf
SSGMCE-Shri Sant Gajanan Maharaj College of Engineering
Logistic management
notes of MBA
BMSCE-B M S College of Engineering
human resource management
I want human resource management notes of 2nd sem.
AIET-Alvas Institute of Engineering and Technology
notes
send me the notes
GU-Galgotias University Noida
Hr and marketing
Hr and marketing manager

NIU-Noida International University
Business Communication
Operationit Mainly deals with communicational prospect that used while working in a company like emails formal letters and Memo
DLRCPGC-DLR College PG Courses
PG
Dyffryn g

ICFAI-ICFAI University
Accounting for Decision Making ...
Accounting for Decision Making is the paper of the MBA Degree under the discipline of accountingt from the ICFAI University.The questions are purely objective and is for a total of 100 marks. There are 72 questions in all. The candidate is required to answer all the questions and time allocated in this paper in 3 hours. The questions are divided into two sections.<br />
XIMB-Xavier Institute of Management Bhubaneswar
Basics of Accounting
https://www.youtube.com/channel/UCIY1EPeMGXTYEBlNMUBLQ8g

AMSSOI-Andhra Mahila Sabha School of Informatics
Managerial Communication notes ...
<strong>Managerial Communication</strong><br /> <br /> A manager can considerably impact workforce expansion and employee performance. Whether such impact is positive or negative, it is often the direct result of communication management and their understanding of each other's work habits and style. In order to understand what communication is to a good manager, let us first describe what communication is and how a manager can benefit through such skills. Communication is the ability to articulate yourself so that others can understand both your words and what your goals are. You have more ways to communicate today than ever before, and many more ways are on the way. Earlier as a manager, you had only a few different communications skills to master in order to be a good manager. Telephones, letters, face-to-face conversations, and the occasional speech or presentation were all about it. Now however, you have all kinds of exciting and new ways to tell your counterpart on the other side of the world to take a walk. You have e-mail both on local networks within companies and on the internet voice mail, voice pagers, conference calls, teleconferencing, faxes, wireless phone, satellite uplinks, satellite downlinks, and on and on. Those are the technology side, but here we are going to focus the ability of a manager to communicate his employers personally. therefore we are going to go through some of those skills that manager is to have, so that he or she can be a good manager, Body communication, Listening communication, Open door policy communication. These are very important in all areas of life, especially the workplace. The communication equation has two sides the doing side and the listening side. Therefore the good manager has to marginalize both qualities in order this equation to be balanced equation.
MIETEC-M I E T Engineering College Trichy
project management
i need study material for project management

ICFAI-ICFAI University
International Management Exam ...
International management is the paper of the MBA Degree under the discipline of international management from the ICFAI University.The mark value of the sections is 30, 50, and 20 for section A, B, and C respectively.<br /> Section A is objective type question, section B is paragraph comprehensive type question and section C has 2 eassy type questions.

ICFAI-ICFAI University
Managerial Effectiveness I MB1 ...
Managerial Effectiveness is the paper of the MBA Degree under the discipline of MBA from the ICFAI University.There are 100 questions in the MBA Managerial Effectiveness – I (MB1A3) Paper and it is a compulsory paper. There are various types of question pattern like fill in the blanks, true false questions. It is 100 marks and it is an objective paper and has one mark each. A time of 3 hours is allocated to answer.<br />
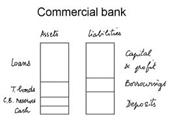
ICFAI-ICFAI University
Central Banking and Commercial ...
Central Banking and Commercial Banking is the paper of the MBA Degree under the discipline of Central Banking and Commercial Banking from the ICFAI University.The exam of this subject is of three hours and the maximum marks are 100. There are three sections in the paper. The first section has MCQs, carrying one mark each. The second section has case studies. Three cases are given and this section is of 50 marks in total. 8 questions are there in this section and last section is of 20 marks which is fully descriptive.<br />

ACACS-Abhinav College of Arts Commerce and Science
MBA 1st year Managerial Econo ...
Managerial Economics is the paper of MBA, 1st year in Bharathiar university.The paper consists of a total of 100 marks and the time allotted for its completion is 3 hours. The paper consist of 8 questions out of which 5 must be answered.Managerial Economics paper is a purely non-technical one and this paper contains 5 very long answer type questions.

ICFAI-ICFAI University
Business Policy and Strategy M ...
Business Policy and Strategy is the paper four of the MBA Degree under the discipline of business studies from the ICFAI University. The paper is for a total of 80 marks. The question paper is divided into two parts and there are both objective and subjective questions in the paper. First part carries 30 marks while the second carry 50 marks.

NIU-Noida International University
Managerial Economics
Study of Economical statistics of the Management regarding the profit, Sales Maximization etc

BEC-Bapatla Engineering College
CLOUD COMPUTING basic
You might have seen network diagrams with a puffy cloud representing the behind-the-scenes components. The cloud conveys the notion that you don’t really need to know the location or number of servers delivering the service. Your experience is that you request a service

KSOU-Karnataka State Open University
MBA First Semester Accounting ...
This is sample paper of MBA 1st sem of Accounting for Managers. The sections of the sample paper is as under:<br /> • Management Theory & Practice<br /> • Managerial Economics<br /> • Accounting for Managers<br /> • Organizational Behavior<br /> • Quantitative Techniques<br /> • Business Ethics & Values<br /> <br />
LPU-Lovely Professional University
Object Oriented Cplus plus by Robert Lafore
Object Oriented C plus plus by Robert Lafore
Latest Study Materials
POST BLOG
Why not help your peers? Share your college’s study materials, lecture notes or assignments
What is there for you?
-
Study material
Colleges are sharing lecture notes, study material, file, assignment etc.

-
Clarify Doubts
Ask any study related doubt and get answered by college students and faculty.

-
Educational videos
College student sharing a great video to help peers study.

-
Previous Year Papers
Access previous year papers for various courses.

-
Info. update by students of the respective college only.
Only student or educator can post study materials.







